Childhood maltreatment, which includes experiences of physical and emotional abuse and neglect, and sexual abuse (McLaughlin, Reference McLaughlin2016; Teicher & Samson, Reference Teicher and Samson2016) is associated with negative mental health outcomes (Beal et al., Reference Beal, Wingrove, Mara, Lutz, Noll and Greiner2018; Herrenkohl, Hong, Klika, Herrenkohl, & Russo, Reference Herrenkohl, Hong, Klika, Herrenkohl and Russo2013). Adolescence is a period of rapid brain development (Larsen & Luna, Reference Larsen and Luna2018), and the experience of maltreatment has been associated with alterations in brain structure during this period, particularly in stress-sensitive brain regions and circuits (Romeo, Reference Romeo2013, Reference Romeo2017). These maltreatment-associated alterations may be formative in contributing to the emergence of psychopathology, particularly depression and anxiety (Romeo, Reference Romeo2013, Reference Romeo2017). However, longitudinal research is yet to characterize adolescent neurodevelopmental alterations that occur following maltreatment and precede mental illness (McCrory, Gerin, & Viding, Reference McCrory, Gerin and Viding2017; McCrory & Viding, Reference McCrory and Viding2015). Given the high prevalence of childhood maltreatment in the general community (Scher, Forde, McQuaid, & Stein, Reference Scher, Forde, McQuaid and Stein2004), investigating longitudinal associations of maltreatment with neurodevelopment of stress-related neural circuitry and depression and anxiety is crucial (McLaughlin, Weissman, & Bitrán, Reference McLaughlin, Weissman and Bitrán2019; Paquola et al., Reference Paquola, Bennett, Hatton, Hermens, Groote and Lagopoulos2017).
The amygdala and hippocampus are stress-sensitive subcortical brain regions (Giedd & Rapoport, Reference Giedd and Rapoport2010) that are critical for emotion and fear processing, emotion regulation, and stress reactivity (Davis, Reference Davis1992; Phelps & LeDoux, Reference Phelps and LeDoux2005). Given the aforementioned functions of these regions, and the fact that they are consistently implicated in the development of depressive and anxiety disorders (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Koolschijn, van IJzendoorn, Bakermans-Kranenburg, & Crone, Reference Koolschijn, van IJzendoorn, Bakermans-Kranenburg and Crone2013; Merz, He, & Noble, Reference Merz, He and Noble2018), the amygdala and hippocampus have been a focus of maltreatment research. Studies in adults have consistently found childhood maltreatment to be associated with volumetric reductions in the hippocampus (Teicher & Samson, Reference Teicher and Samson2016). However, findings in adolescents have been inconclusive, with studies reporting both increases (Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013) and decreases (Redlich et al., Reference Redlich, Opel, Bürger, Dohm, Grotegerd, Förster and Dannlowski2018) in hippocampal volume as a function of maltreatment, as well as some findings of no differences (de Brito et al., Reference de Brito, Viding, Sebastian, Kelly, Mechelli, Maris and McCrory2013; Korgaonkar et al., Reference Korgaonkar, Antees, Williams, Gatt, Bryant, Cohen and Grieve2013). Similarly, studies of amygdala volume in youth with maltreatment history have also yielded mixed findings (Paquola, Bennett, & Lagopoulos, Reference Paquola, Bennett and Lagopoulos2016).
Inconsistent findings in the literature may be due to small sample sizes and cross-sectional designs. Unlike cross-sectional studies, longitudinal studies provide an opportunity to mitigate cohort effects and to investigate within-subject change in amygdala and hippocampal volume across development. Longitudinal literature on maltreatment-related changes in subcortical morphology during adolescence is scarce; however, existing studies do provide some evidence for effects of maltreatment on developmental trajectories of both the amygdala and hippocampus. Two studies found that maltreatment was associated with flatter amygdala growth from early to mid-adolescence (Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013) and during late childhood (VanTieghem et al., Reference VanTieghem, Korom, Flannery, Choy, Caldera, Humphreys and Tottenham2021). Studies have also reported flatter hippocampal growth from early to mid-adolescence (Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013) and between ages 14–28 (Paquola et al., Reference Paquola, Bennett, Hatton, Hermens, Groote and Lagopoulos2017).
Moreover, the amygdala and hippocampus have complex interactions with other brain regions (particularly the prefrontal cortex [PFC]) to carry out social, affective, and cognitive functions. The PFC is considered important for emotion generation and regulation, as well as fear learning and extinction through executive control over the amygdala and hippocampus (Dixon, Thiruchselvam, Todd, & Christoff, Reference Dixon, Thiruchselvam, Todd and Christoff2017; McEwen, Reference McEwen2005; McGarry & Carter, Reference McGarry and Carter2017; Pattwell et al., Reference Pattwell, Liston, Jing, Ninan, Yang, Witztum and Lee2016; Yurgelun-Todd & Killgore, Reference Yurgelun-Todd and Killgore2006). Interactions between the amygdala/hippocampus and the PFC have also shown to be implicated in studies of childhood maltreatment. The development of PFC interactions with subcortical regions has been suggested to be impacted by early life stress (Calabro, Murty, Jalbrzikowski, Tervo-Clemmens, & Luna, Reference Calabro, Murty, Jalbrzikowski, Tervo-Clemmens and Luna2020; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016). For example, studies have shown that previously institutionalized youth showed greater hippocampus-ventromedial PFC connectivity, resembling a more adult-like phenotype (Calabro et al., Reference Calabro, Murty, Jalbrzikowski, Tervo-Clemmens and Luna2020; Silvers et al., Reference Silvers, Lumian, Gabard-Durnam, Gee, Goff, Fareri and Tottenham2016) suggesting that stress-related changes in neurodevelopmental trajectories of subcortical-cortical circuits may be relevant in the context of maltreatment. However, few studies have tested this hypothesis using measures of brain structure. Investigating structural maturational coupling may be fruitful in this regard.
Analysis of structural maturational coupling allows investigations of patterns of longitudinal change between brain regions as they mature (Alexander-Bloch, Raznahan, Bullmore, & Giedd, Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013). Correlated change may indicate regions developing in a coordinated manner (Alexander-Bloch et al., Reference Alexander-Bloch, Raznahan, Bullmore and Giedd2013). Positive and negative maturational coupling represents coordinated development in the same and opposite directions, respectively (Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017). Subcortico-cortical maturational coupling has been shown to be relevant for mental health. Vijayakumar et al. (Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017), for example, found that stronger positive right amygdala-left anterior PFC maturational coupling was associated with decreases in depressive symptoms across adolescence. Roberts et al. (Reference Roberts, Pozzi, Vijayakumar, Richmond, Bray, Deane and Whittle2021) reported that reductions in aggressive behaviour were associated with stronger positive left amygdala-cortical maturational coupling in children. While associations between maltreatment and functional connectivity development during adolescence have been investigated (Rakesh, Allen, & Whittle, Reference Rakesh, Allen and Whittle2021a; Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b; Yurgelun-Todd & Killgore, Reference Yurgelun-Todd and Killgore2006), less is known about subcortico-cortical structural maturational coupling in this context. Indeed, no studies to our knowledge have investigated maltreatment and longitudinal changes in structural maturational coupling. However, given the role that subcortico-cortical maturational coupling may play in mental health, understanding maltreatment's relationship with subcortico-cortical maturational coupling, and in turn, mental health during adolescence is imperative.
The main aim of this longitudinal study was to investigate the association between childhood maltreatment and (1) amygdala and hippocampal development, and (2) subcortical-PFC coupling across adolescence. Based on prior literature (Paquola et al., Reference Paquola, Bennett, Hatton, Hermens, Groote and Lagopoulos2017; VanTieghem et al., Reference VanTieghem, Korom, Flannery, Choy, Caldera, Humphreys and Tottenham2021; Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013), we hypothesized that childhood maltreatment would be associated with attenuated development of the amygdala and hippocampus during adolescence. Given that previous literature has shown a relationship between stronger positive subcortical-PFC maturational coupling and decreased mental health symptoms (Roberts et al., Reference Roberts, Pozzi, Vijayakumar, Richmond, Bray, Deane and Whittle2021; Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017), and between greater maltreatment and negative mental health outcomes (Beal et al., Reference Beal, Wingrove, Mara, Lutz, Noll and Greiner2018; Herrenkohl et al., Reference Herrenkohl, Hong, Klika, Herrenkohl and Russo2013), we hypothesized that maltreatment would relate to weaker positive subcortical-PFC maturational coupling.
The secondary aims of this study were (1) to investigate whether dimensions of childhood maltreatment (i.e. deprivation and threat) had differential associations with amygdala and hippocampus development, as well as with subcortico-cortical maturational coupling, and (2) to investigate whether maltreatment-related changes in neurodevelopment mediate the relationship between childhood maltreatment and trajectories of mental health symptoms from early adolescence to early adulthood. Regarding (1), while a cumulative risk approach has predominantly been used in existing literature (Evans, Li, & Whipple, Reference Evans, Li and Whipple2013; McLaughlin, Sheridan, Humphreys, Belsky, & Ellis, Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2020; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014; Steine et al., Reference Steine, Winje, Krystal, Bjorvatn, Milde, Grønli and Pallesen2017), it may be of interest to investigate subtypes of maltreatment, as suggested by the Dimensional Model of Adversity and Psychopathology (DMAP; Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). DMAP emphasizes the importance of distinguishing between dimensions of deprivation (experiences with a lack of expected cognitive and/or social inputs, such as neglect) and threat (unexpected experiences that pose a threat to life or physical safety, such as abuse), and suggest that they may have unique effects on neurodevelopment (Sheridan & McLaughlin, Reference Sheridan and McLaughlin2014). While there has been no work to our knowledge investigating differential effects of abuse and neglect on the development of amygdala and hippocampal volume, or subcortical-PFC maturational structural coupling, based on the theoretical suggestion that threat is more likely to be associated with limbic system functioning (McLaughlin et al., Reference McLaughlin, Weissman and Bitrán2019), we anticipated that we would see effects for abuse but not neglect. Regarding secondary aim (2), we focussed on depressive and anxiety symptoms given particularly strong associations between maltreatment and these mental health outcomes (Li, D'Arcy, & Meng, Reference Li, D'Arcy and Meng2016). We did not make specific hypotheses for this exploratory aim due to the paucity of literature on the subject.
Methods
Participants
Participants were from the Orygen Adolescent Development Study (OADS). 415 participants (from a representative screening sample of 2479 children from schools in Metropolitan Melbourne) were selected to represent the full range of temperamental risk and resilience for depression (Whittle et al., Reference Whittle, Yücel, Fornito, Barrett, Wood, Lubman and Allen2008). Of these, 245 adolescents consented to participate in longitudinal research. Prior to their inclusion in the study, informed consent was obtained from both the participants and their guardians, as per the requirements of The University of Melbourne's Human Research Ethics Committee (Melbourne, Australia). The participants’ demographic information, such as sex, date of birth, and home address was collected. Socioeconomic Status (SES) was assessed using the Index for Relative Socio-economic Disadvantage score based on Australian census data (Pink, Reference Pink2008). A short form of the Wechsler Intelligence Scale for Children IV using the Vocabulary, Matric Reasoning and Symbol Search parameters, was used to estimate IQ at time 1 (T1; Wechsler, Reference Wechsler2003). Participants were assessed using structural Magnetic Resonance Imaging (MRI) at three time points across adolescence (T1, T3, T4; see Table 1 for demographic information); 166 participants had imaging data available from at least one time point. Mental health surveys were completed at the same time points. Additionally, 114 participants completed a follow-up mental health survey during early adulthood (T5). Childhood maltreatment data was collected at T2. The final sample size for primary analyses was 144 (all participants that had MRI and symptom data from at least one time point, and childhood maltreatment data). Of these 144 participants, 27 had one usable scan, 48 had two scans, and 69 had three usable scans. See online Supplementary Figure S1 for measures taken across T1–T5.
Table 1. Demographic, maltreatment score, and symptomatology descriptive statistics

T1–T5 refer to time points 1–5. Brain imaging was completed at T1, T3 and T4. N refers to the number of participants who completed brain imaging at T1, T3 and T4, and CTQ at T2. N (CES-D and BAI) refers to the number of participants who completed the CESD and BAI questionnaires at T1, T3, T4 and T5. Statistics shown as mean ± standard deviation.
BAI, Beck Anxiety Inventory; CES-D, Centre for Epidemiological Studies-Depression scale; CTQ, Childhood Trauma Questionnaire; SES, socioeconomic status.
Childhood maltreatment measure
The Childhood Trauma Questionnaire (CTQ) was administered at age 14 (T2) and participants reported on childhood maltreatment that occurred prior to the first imaging scan. The CTQ is a 28-item retrospective self-report measure that assesses five maltreatment subtypes of physical and emotional abuse and neglect, as well as sexual abuse. Each subscale comprises 5 items, and there is a minimization/denial scale, which comprises 3 items. Participants respond to each item via a Likert scale, with response options from 1 (never true) to 5 (very often true). Total maltreatment was calculated by summing all items in the CTQ. Total neglect was calculated by summing physical and emotional neglect scores, while total abuse was calculated by summing physical and emotional abuse scores. The inclusion of sexual abuse in the total abuse score decreased its internal consistency due to only three adolescents endorsing experiences of sexual abuse. Specifically, the Cronbach α of neglect (physical + emotional neglect) was 0.83, whereas the value for abuse (physical + emotional + sexual) was low (α = 0.3). This value was found to be 0.8 when sexual abuse was excluded. Thus, sexual abuse was excluded from the analyses of abuse in this study. Abuse and neglect scores were moderately correlated (Pearson r = 0.495).
Participants responded to each question by choosing a number between one (never true) and five (very often true) to reflect the frequency of the experience(s). They are also asked to indicate at what age maltreatment was experienced. Given our aim of investigating the effects of maltreatment that occurred during childhood and prior to the first MRI assessment, we scored items only if participants indicated that the experience occurred or began prior to T1 (i.e. prior to calculating total/subscale scores, any item that was rated as occurring, or beginning after the age of the participant's T1 assessment was recoded as ‘never true’ (or ‘very often true’ in the case of reverse-coded items). The internal consistency of total maltreatment, total neglect, and total abuse scores were acceptable (Cronbach α: 0.76, 0.83 and 0.8, respectively).
Symptom measures
At T1, T3, T4, and T5, anxious and depressive symptomatology was assessed using the Beck Anxiety Inventory (Beck, Epstein, Brown, & Steer, Reference Beck, Epstein, Brown and Steer1988) and the Centre for Epidemiological Depression (CESD) Scale (Radloff, Reference Radloff1977). The BAI is a 21-item self-report tool to determine presence and severity of anxiety symptoms in clinical and non-clinical populations (Beck et al., Reference Beck, Epstein, Brown and Steer1988). Similarly, the CESD is a 20-item self-report tool that assesses depressive symptom severity (Radloff, Reference Radloff1977). Internal consistency values for CESD and BAI at each time point and Pearson correlation values for abuse/neglect and CESD and BAI scores at each time point have been provided in online Supplementary Material.
MRI acquisition and processing
Scans were conducted on either Signa Horizon LX Human (General Electric Company; T1) or MAGNETOM Trio (Siemens; T3 and T4) at the Royal Children's Hospital (Melbourne, Australia). See online Supplementary Material for acquisition parameters, quality control, and analyses on inter-scanner reliability. Structural MRI scans were processed using the longitudinal stream in FreeSurfer version 5.3. Data for subcortical volume and cortical thickness was then extracted using the subcortical and Desikan-Killiany parcellation schemes, respectively, and used in analyses. Of 34 cortical and 7 subcortical parcellations available in FreeSurfer, the volume of two subcortical regions (amygdala and hippocampus), and the thickness of ten prefrontal cortical regions (caudal anterior cingulate, rostral anterior cingulate, caudal middle frontal, rostral middle frontal, superior frontal, lateral orbitofrontal, medial orbitofrontal, pars opercularis, pars orbitalis and pars triangularis) were utilized in this study. Measures from left and right hemisphere were averaged given we had no hypotheses regarding lateralization.
Statistical analyses
The lme4 package in R (version 1.4.1717) was used to conduct linear mixed model (LMM) analyses to investigate associations between maltreatment and brain development. LMMs allow for the analysis of repeated measures data, and for the use of all available data (including participants with data from only one and two time-points) (Gibbons, Hedeker, & Dutoit, Reference Gibbons, Hedeker and Dutoit2010). There were a maximum of three neuroimaging data points per participant, thus, in order to prevent over-fitting the data we investigated brain development linearly rather than using quadratic or cubic functions (Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017). The package lmerTest was utilized to obtain p values.
Primary analyses
To be able to comment on maltreatment-associated patterns, we first examined normative developmental trajectories of amygdala and hippocampus volume across the whole sample. Next, we modelled a two-way interaction between maltreatment and age to investigate whether maltreatment was associated with amygdala and hippocampal development (in separate models) from age 12 to 19. Finally, to investigate the role of maltreatment in shaping subcortico-cortical maturational coupling, we modelled a three-way interaction between CTQ scores, age and change in hippocampus/amygdala volume (operationalized as random slopes extracted from LMMs; see online Supplementary Material and Table S9 for details) predicting prefrontal thickness. The nature and direction of maturational coupling that emerged from these analyses were described and interpreted based on previous studies (Roberts et al., Reference Roberts, Pozzi, Vijayakumar, Richmond, Bray, Deane and Whittle2021; Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017). That is, we interpreted maltreatment-related increases and/or decreases in subcortical and PFC structure with age relative to normative patterns. For example, because normative development was characterized by age-related increases in amygdala volume and decreases in PFC thickness (see below Results), a maltreatment-related increase in amygdala volume coupled with a decrease in PFC thinning (i.e. relative increase in PFC thickness) would be interpreted as maltreatment-related increase in positive maturational coupling. See online Supplementary Material for further explanation and an example figure (online Supplementary Figure S2).
We covaried for sex, SES, and IQ in all models, and all variables were standardized for analyses. Results controlling for total intracranial volume (TIV) can be found in online Supplementary Material. We corrected for multiple comparisons within total CTQ, abuse, and neglect models (i.e. two comparisons for subcortical development, and ten comparisons each for hippocampal- and amygdala-cortical coupling analyses) using the False Discovery rate (FDR) method (p < 0.05) (Benjamini & Hochberg, Reference Benjamini and Hochberg1995). See online Supplementary Material for model equations. To ensure that findings were not affected by non-normal distributions of model residuals, all significant associations were tested further using robust linear mixed models using the robustlmm and lme4 packages. To probe any observed interaction effects, data were divided into individuals with relatively high and low maltreatment exposure using a median split of CTQ (total/abuse/neglect) scores.
Secondary analyses: abuse and neglect
We examined effects of abuse and neglect (in separate models) in the same manner as described above. In sensitivity analyses, we controlled for neglect-associated change (e.g. neglect × age) in abuse models and abuse-associated change in neglect models.
Secondary analyses: sex
We investigated sex as a moderator of maltreatment-brain development associations (see online Supplementary Material for details).
Secondary analyses: mediation models
In addition, for significant associations identified from the aforementioned analyses (i.e. total maltreatment, abuse, and neglect), mediation analyses were run to determine if these relationships were associated with depression and anxiety symptom trajectories. For significant subcortical-cortical coupling analyses, we tested whether prefrontal development mediated the relationship between maltreatment and change in depression and anxiety symptoms and whether amygdala/hippocampal change slopes moderated these relationships using moderated mediation models. To this end, LMM analyses were run to investigate age-related change in symptoms, and random slopes for age were then extracted to reflect symptom change over time. These scores (i.e. random slopes) were then used as outcome variables in the mediation analyses (see online Supplementary Material, Figures S3 and S4 for further details). Covariates for mediation analyses were IQ, SES, and sex. Mediation analyses were implemented using the PROCESS macro for R.
Results
Demographic information and descriptive statistics for the sample can be found in Table 1. Participants were aged 11.5–13.9 years at baseline, 15.2–18.3 years at the second imaging time-point, and 17.5–20.3 years at the third imaging time-point. The average time between the first and second imaging time-point was 3.82 years (range 3.35–4.63 years), and between the second and third time point was 2.67 years (range 1.7–2.93 years).
Normative amygdala and hippocampus development
There was a significant effect of age on amygdala and hippocampal volumes. Although sex effects were not of primary interest in maltreatment analyses, sex was tested as a moderator in normative models (Wierenga et al., Reference Wierenga, Langen, Ambrosino, van Dijk, Oranje and Durston2014). Sex significantly moderated the effect of age on both amygdala and hippocampal volumes such that males exhibited greater normative increases between ages 11 and 20 than females (see online Supplementary Table S1, Figure S5).
Relationships between total maltreatment and amygdala and hippocampus development
An interaction between maltreatment and age did not significantly predict amygdala or hippocampus volume (p values >0.05). See online Supplementary Table S2 for model output.
Relationships between total maltreatment and subcortical-cortical maturational coupling
A significant three-way interaction between total maltreatment, age, and amygdala random slope significantly predicted caudal anterior cingulate (cACC) thickness (B = 0.6, s.e. = 0.19, t = 3.14, p = 0.002; see Fig. 1 and Table 2 for model output). To disentangle effects, we probed the interaction by conducting analyses within low and high maltreatment exposure groups (based on a median split of the total CTQ score). A two-way interaction between age and amygdala random slope significantly predicted cACC thickness in those with high maltreatment exposure, such that reduced amygdala growth was associated with relatively increased cortical thinning (positive coupling). This effect was not present in individuals with low maltreatment exposure. No other significant associations were found between total maltreatment and subcortical-cortical maturational coupling (pFDR > 0.05, see online Supplementary Tables S3 and S4 for model output for non-significant associations).

Fig. 1. Maltreatment-associated developmental trajectories of amygdala-caudal anterior cingulate coupling. (A) Cortical rendering highlights the caudal anterior cingulate region, which showed significant positive coupling with the amygdala, with the t statistic (3.14). Only one hemisphere is visualized as we used mean bilateral thickness measures. Amygdala-caudal anterior cingulate maturational coupling between the ages of 12 and 19 at (B) high and (C) low CTQ scores. Statistics reported in Table 2 and Table S3. Slopes represent average trajectories for +1SD, mean, and −1SD of amygdala random slopes. (* = significant age by amygdala random slope interaction, p < 0.05).
Table 2. Significant associations between maltreatment (total and neglect) and brain development (subcortical development and subcortical-cortical maturational coupling)
SE, Standard Error.
RS, Random Slope.
*pFDR < 0.05.
Note. Non-significant results in Supplementary Material.
1 All variables in this column are time-varying.
Secondary analyses
Associations between abuse/neglect and brain development
While abuse and neglect were not significantly associated with amygdala or hippocampus development (see online Supplementary Table S2 for model output), there was a significant three-way interaction between sex, neglect, and age when predicting amygdala volume (B = 0.102, s.e. = 0.04, t = 2.91, p = 0.004). The association between neglect and amygdala development was significant in females but not males. In females, higher neglect was associated with greater increases in amygdala volume with age (see online Supplementary Figure S7).
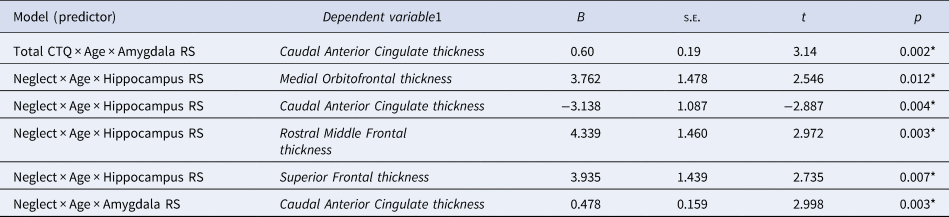
In addition, neglect was found to be significantly associated with maturational coupling of the hippocampus with several prefrontal regions (see online Supplementary Fig. 2-3, Table S6). Specifically, neglect was associated with maturational coupling between the hippocampus and medial orbitofrontal (B = 3.76, s.e. = 1.48, t = 2.55, p = 0.012), caudal anterior cingulate (B = −3.13, s.e. = 1.09, t = −2.89, p = 0.004), rostral middle frontal (B = 4.4, s.e. = 1.46, t = 2.97, p = 0.003), and superior frontal thickness (B = 3.94, s.e. = 1.44, t = 2.74, p = 0.007). Further, neglect was associated with maturational coupling of the amygdala and caudal anterior cingulate thickness (B = 0.48, s.e. = 0.16, t = 3, p = 0.003; Figure 4).
Follow-up analyses showed that increased hippocampal growth was significantly associated with greater cortical thinning (negative coupling) in the cACC in the high but not the low neglect group (Fig. 2). Conversely, in those with high neglect exposure, relatively decreased hippocampal growth was significantly associated with relatively increased cortical thinning (positive coupling) in the caudal middle frontal, rostral middle frontal, and superior frontal regions (Fig. 3). Similarly, high neglect exposure was associated with the coupling of low amygdala growth with relatively increased cortical thinning in the caudal anterior cingulate (positive coupling; Figure 4). While there was also an overall significant association between neglect and hippocampus-cortical maturational coupling for medial orbitofrontal thickness, effects were not significant at high or low neglect exposure (likely due to reduced power). Neglect was not associated with maturational coupling of the amygdala/hippocampus with any other prefrontal regions and sex was not found to moderate any associations (online Supplementary Tables S5 and S6; pFDR > 0.05). Further, abuse was not associated with maturational coupling of the amygdala (online Supplementary Table S7) or hippocampus (online Supplementary Table S8) with prefrontal regions and sex did not significantly moderate any associations (pFDR > 0.05). Importantly, results remained unchanged in sensitivity analyses where we included neglect-associated change in abuse models and abuse-associated change in neglect models (online Supplementary Table S17).

Fig. 2. Neglect-associated maturational trajectories of hippocampal-caudal anterior cingulate coupling. (A) Cortical rendering highlights the caudal anterior cingulate region, which showed significant negative coupling with the hippocampus, with the t statistic (−2.84). Only one hemisphere is visualized as we used mean bilateral thickness measures. (B and C) This figure depicts change in caudal anterior cingulate thickness over time at different levels of change in hippocampal volume (slopes represent trajectories for +1SD, mean, and −1SD of hippocampus random slopes) in individuals who have experienced high (B) and low (C) levels of neglect. At high (but not low) levels of neglect there is negative maturational coupling of the hippocampus and caudal anterior cingulate (* = significant age by hippocampus random slope interaction, pFDR < 0.05).
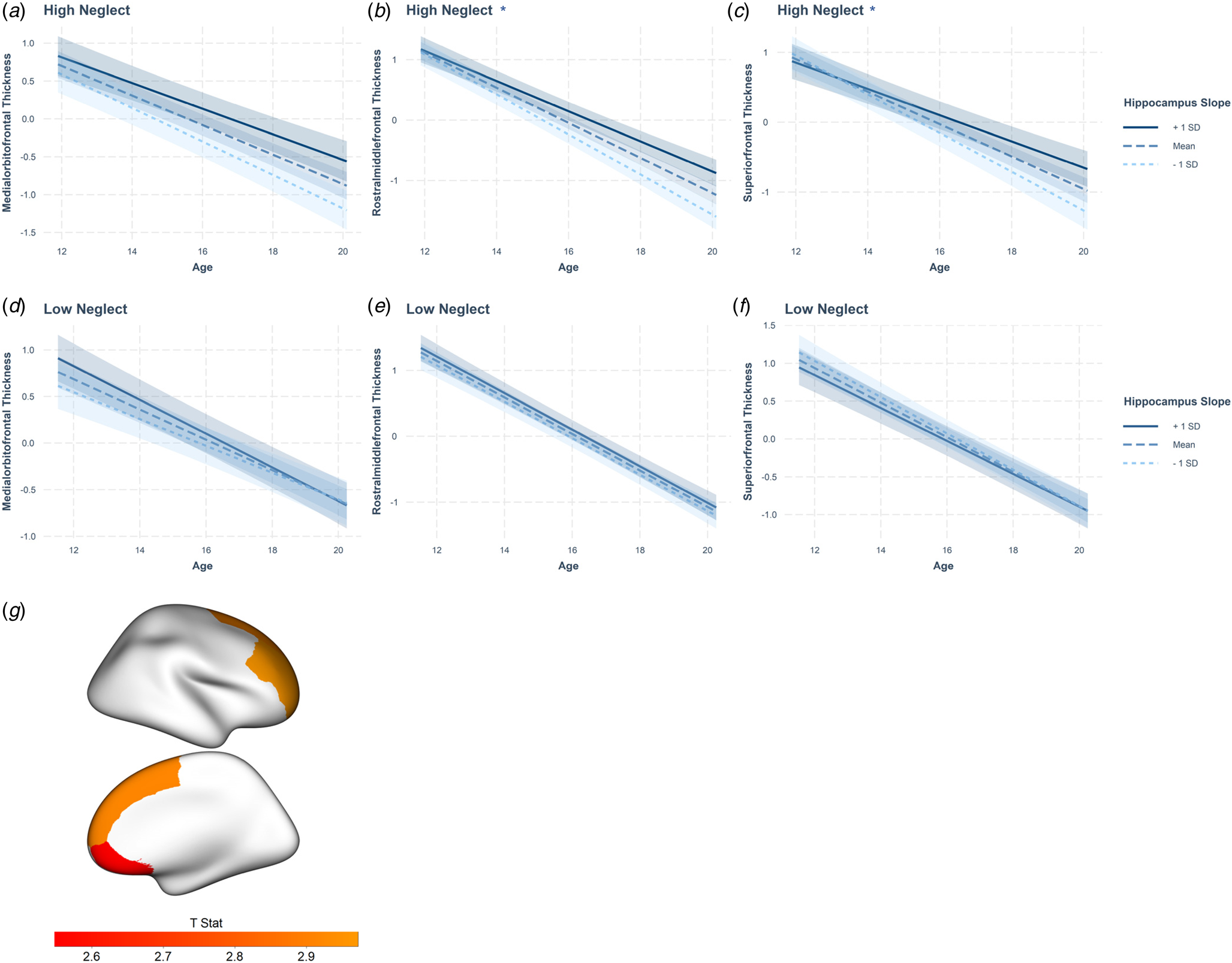
Fig. 3. Neglect-associated maturational trajectories of hippocampal-prefrontal coupling. This figure depicts change in prefrontal thickness over time at different levels of change in hippocampal volume (slopes represent average trajectories for +1SD, mean, and −1SD of hippocampus random slopes) in individuals who have experienced low and high levels neglect for the medial orbitofrontal (A, D), rostral middle frontal (B, E), and superior frontal (C, F) regions. Across all three cortical regions, at high (but not low) levels of neglect, there is positive maturational coupling of the hippocampus and prefrontal regions (although the effect was not significant in those with high neglect for the medial orbitofrontal cortex). (G) Cortical rendering highlights cortical regions that showed significant positive coupling with the hippocampus. Colour bar depicts t statistics for each region.
* = significant age by hippocampus random slope interaction.
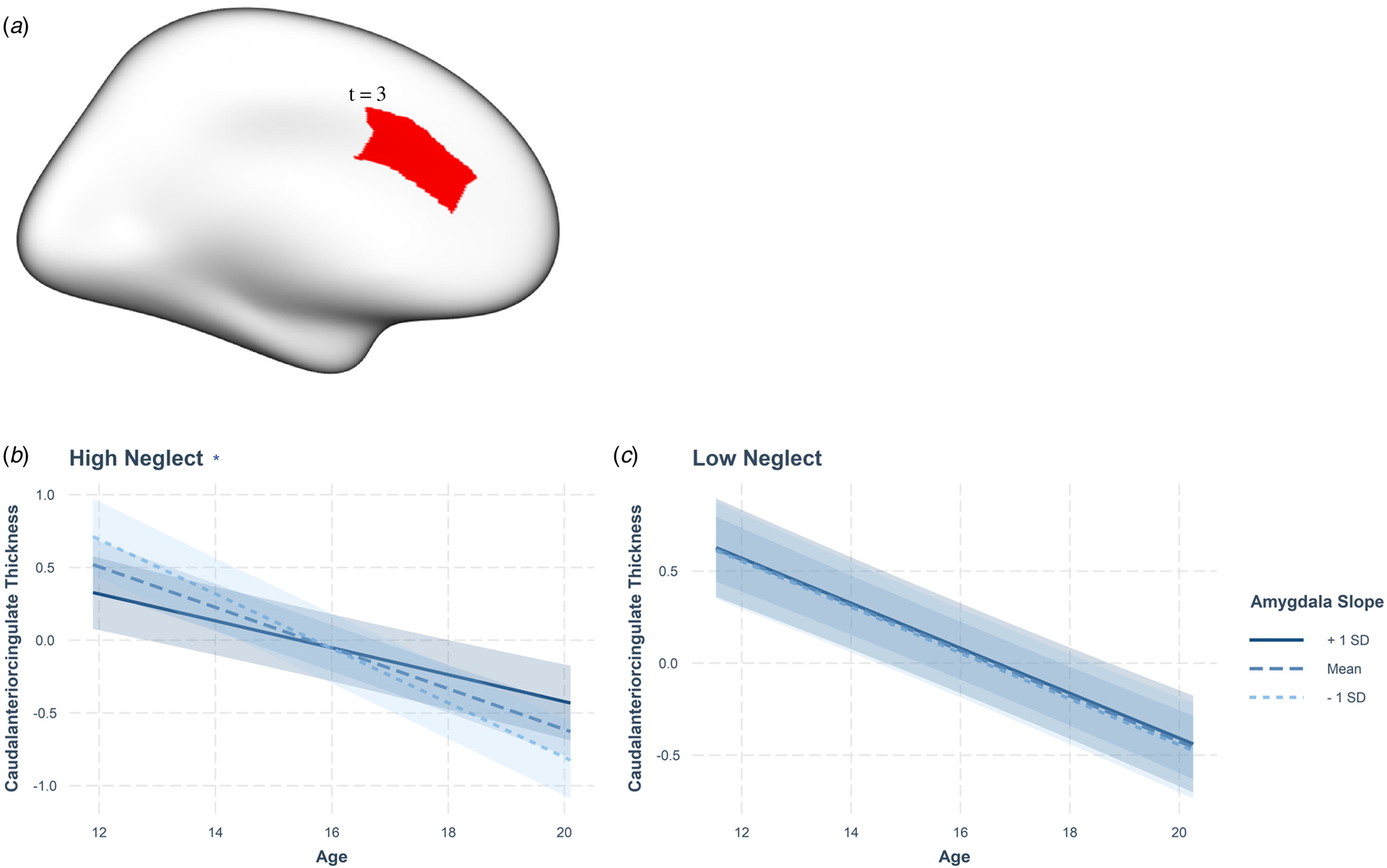
Fig. 4. Neglect-associated developmental trajectories of amygdala-caudal anterior cingulate coupling. (A) Cortical rendering highlights the caudal anterior cingulate region, which showed significant positive coupling with the amygdala, with the t statistic (3). Only one hemisphere is visualized as we used mean bilateral thickness measures. Amygdala-caudal anterior cingulate maturational coupling between the ages of 12 and 19 at (B) high and (C) low neglect scores. Statistics reported in Table 2 and Table S5. Slopes represent average trajectories for +1SD, mean, and −1SD of amygdala random slopes. (* = significant age by amygdala random slope interaction, p < 0.001).
Mediation models
There were no moderated mediation effects present for any neurodevelopmental variables implicated in the aforementioned analyses for either depressive or anxiety symptom trajectories (see online Supplementary Tables S10–S14). However, maturational coupling of the amygdala and cACC significantly predicted greater increases in BAI scores (p = 0.012 and p = 0.015, see online Supplementary Figure S6 and S7).
All significant associations that survived FDR testing were also significant using robust LMM (see online Supplementary Table S16 for model outputs).
Discussion
In this longitudinal investigation of associations between childhood maltreatment and brain development during adolescence, we found that higher neglect exposure was associated with positive maturational coupling of the hippocampus and several prefrontal regions (rostral middle frontal, medial orbitofrontal, and superior frontal regions), and negative coupling of the hippocampus with the cACC during adolescence. Further, we found that higher total maltreatment and neglect specifically, were significantly associated with positive maturational coupling between the amygdala and cACC. While this pattern was associated with an increase in anxiety symptoms, amygdala-cACC maturational coupling did not mediate the association between maltreatment and anxiety symptom development.
Inconsistent with our hypothesis, we did not find maltreatment to be associated with amygdala or hippocampal development. This finding is inconsistent with previous studies reporting maltreatment to be associated with longitudinal reductions in amygdala and hippocampal growth in children and adolescents. However, prior work investigated a restricted adolescent age range (Whittle et al., Reference Whittle, Dennison, Vijayakumar, Simmons, Yücel, Lubman and Allen2013), or utilized an accelerated longitudinal design (VanTieghem et al., Reference VanTieghem, Korom, Flannery, Choy, Caldera, Humphreys and Tottenham2021), which may not accurately represent the trajectory of brain development across the age group examined in our study (12–20 years old) (Galbraith, Bowden, & Mander, Reference Galbraith, Bowden and Mander2017; VanTieghem et al., Reference VanTieghem, Korom, Flannery, Choy, Caldera, Humphreys and Tottenham2021). Differences between studies in exposure type and timing may have also contributed to inconsistent findings (Herzog et al., Reference Herzog, Thome, Demirakca, Koppe, Ende, Lis and Schmahl2020). Of note, however, exploratory analyses revealed greater increases in amygdala development in females who had experienced higher levels of neglect. Given that we found normative age-related increases in amygdala volume in females, our results may reflect accelerated development of the amygdala as a function of neglect only in females (sex-specificity discussed further in online Supplementary Material).
We found that higher total maltreatment/neglect exposure was significantly associated with positive maturational coupling between the amygdala and the cACC (i.e. in those with high maltreatment/neglect exposure, the relative direction of development in both regions was the same). In those with low maltreatment exposure, the development of these two regions was independent of one another (i.e. it was not ‘coupled’). Two comparable longitudinal studies suggested that positive maturational coupling (between the amygdala and several cortical regions, not including the cACC) represented a normative pattern of development as it was associated with relative reductions in aggression and depressive symptoms (Roberts et al., Reference Roberts, Pozzi, Vijayakumar, Richmond, Bray, Deane and Whittle2021; Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017).
The present study identified positive maturational coupling between the amygdala and cACC at higher levels of maltreatment. Secondary analyses showed that this finding was driven by neglect and not abuse. High levels of maltreatment/neglect reflect a stressful, non-normative developmental environment. Based on the suggestion that positive coupling may reflect normative coordinated development (Roberts et al., Reference Roberts, Pozzi, Vijayakumar, Richmond, Bray, Deane and Whittle2021; Vijayakumar et al., Reference Vijayakumar, Allen, Dennison, Byrne, Simmons and Whittle2017), our findings may reflect an acceleration of this pattern as a function of neglect. This interpretation may be in line with the Stress Acceleration Hypothesis (Callaghan & Tottenham, Reference Callaghan and Tottenham2016), which states that accelerated maturation of stress-regulatory mechanisms likely increase an individuals’ chances of survival under adverse circumstances. However, very little is known about normative patterns of coordinated development of different brain structures during adolescence. In addition, findings of links between adversity and accelerated brain development are not always consistent (Colich, Rosen, Williams, & McLaughlin, Reference Colich, Rosen, Williams and McLaughlin2020; Rakesh & Whittle, Reference Rakesh and Whittle2021). Further, our findings could also be interpreted as maltreatment being associated with the coupling of faster and slower development, either (1) faster amygdala growth and attenuated pruning of prefrontal regions, or (2) slower amygdala growth and faster thinning of the prefrontal regions. As such, interpretations about the association between maltreatment/adversity and the pace of brain development, particularly in the context of coupling, are complicated.
The cACC and amygdala are part of the salience network (Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna and Greicius2007) and are involved in the detection and interpretation of salient information in the environment (Milad et al., Reference Milad, Quirk, Pitman, Orr, Fischl and Rauch2007). Previous research has also reported maltreatment and other types of adversity to be associated with salience network connectivity and activation (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013; Jenness et al., Reference Jenness, Peverill, Miller, Heleniak, Robertson, Sambrook and McLaughlin2021; McLaughlin, Peverill, Gold, Alves, & Sheridan, Reference McLaughlin, Peverill, Gold, Alves and Sheridan2015; Rakesh et al., Reference Rakesh, Allen and Whittle2021a; Weissman et al., Reference Weissman, Jenness, Colich, Miller, Sambrook, Sheridan and McLaughlin2020). Disrupted salience network functional connectivity has also been implicated in various types of anxiety disorders (Pannekoek et al., Reference Pannekoek, Veer, Van Tol, Van Der Werff, Demenescu, Aleman and Van Der Wee2013a, Reference Pannekoek, Veer, Van Tol, Van der Werff, Demenescu, Aleman and Van der Weeb; Xiong, Guo, & Shi, Reference Xiong, Guo and Shi2020). Indeed, in exploratory analyses, we found a significant positive association between positive amygdala-cACC maturational coupling and the change in anxiety symptoms. Thus, we speculate that maltreatment-related alterations to amygdala-cACC maturational coupling are related to the functional development of the salience network, and this may have implications for mental health. However, because amygdala-cACC maturational coupling did not mediate the relationship between maltreatment/neglect and anxiety symptom development, the role of amygdala-cACC circuitry in increased vulnerability for mental illness in adolescents with a history of childhood maltreatment remains uncertain.
Neglect was also found to be associated with positive maturational coupling between hippocampal volume and thickness of the superior frontal, rostral middle frontal, and medial orbitofrontal regions. Given the role that hippocampal-prefrontal connectivity plays in cognitive function (Rasetti & Weinberger, Reference Rasetti and Weinberger2011), we speculate that these alterations may be associated with neglect-associated alterations in cognitive performance (Gould et al., Reference Gould, Clarke, Heim, Harvey, Majer and Nemeroff2012). Further longitudinal research is required to better understand hippocampus-PFC maturational coupling, and its relationship to maltreatment, cognitive function, and mental health.
Interestingly, higher neglect exposure was associated with negative maturational coupling of the hippocampus and the cACC (i.e. opposite relative direction of subcortical and cortical development). Albeit in different directions (i.e. positive and negative), existing research has found abnormal functional maturation and connectivity of hippocampus-PFC circuitry to be associated with vulnerability to psychopathology (Godsil, Kiss, Spedding, & Jay, Reference Godsil, Kiss, Spedding and Jay2013; Hartung et al., Reference Hartung, Cichon, De Feo, Riemann, Schildt, Lindemann and Hanganu-Opatz2016; Meyer-Lindenberg et al., Reference Meyer-Lindenberg, Olsen, Kohn, Brown, Egan, Weinberger and Berman2005; Oberlander, Xu, Chini, & Hanganu-Opatz, Reference Oberlander, Xu, Chini and Hanganu-Opatz2019; Sigurdsson & Duvarci, Reference Sigurdsson and Duvarci2016). Research has also found maltreatment to be associated with altered integrity of white matter tracts connecting the hippocampus and anterior cingulate cortex in those who subsequently develop psychopathology (Huang, Gundapuneedi, & Rao, Reference Huang, Gundapuneedi and Rao2012). Our finding of negative hippocampus-cACC maturational coupling could reflect disrupted or altered development; however, we did not find any significant associations with anxiety or depressive symptoms. As such, the significance of our finding for mental health is unclear.
Hippocampal-PFC maturational coupling did not have any associations with anxiety or depressive symptom trajectories from adolescence through early adulthood. As such, the implications for clinical outcomes are unclear. It is possible that maltreatment-associated neural alterations reported in this study could be associated with mental health domains we did not test (e.g. externalizing behaviour). Further longitudinal studies incorporating other domains of mental health are required to better understand neurodevelopmental resilience to adversity. Alternatively, given that we did not find a risk relationship, and the fact that not everyone who experiences childhood maltreatment has poor mental health (Klika & Herrenkohl, Reference Klika and Herrenkohl2013), it is possible that our hippocampus-PFC findings may reflect a resilience mechanism. That is, changes in neurodevelopment following maltreatment may not necessarily be reflective of ‘damage’, but rather of ‘adaptation’ that may prevent the onset of poor mental health in later years. However, in the absence of an association with lower incidence of symptoms, this idea is speculative and further research is needed.
There were no associations between abuse and brain development, which was inconsistent with our hypotheses. However, given that we found different effects for abuse and neglect, our findings partially support the DMAP. Our study highlights that further nuanced research is required to extend existing frameworks to understand the impact of childhood maltreatment on brain development.
There were some limitations associated with this study. First, the amygdala and hippocampus are heterogeneous regions, each made up of multiple functionally distinct but interdependent subregions (Aghamohammadi-Sereshki et al., Reference Aghamohammadi-Sereshki, Coupland, Silverstone, Huang, Hegadoren, Carter and Malykhin2021; Ahmed-Leitao et al., Reference Ahmed-Leitao, Rosenstein, Marx, Young, Korte and Seedat2019; Sah, Faber, De Armentia, & Power, Reference Sah, Faber, De Armentia and Power2003). Childhood maltreatment and trauma have been found to influence volumes of some hippocampal subfields and amygdala sub-nuclei but not others (Aghamohammadi-Sereshki et al., Reference Aghamohammadi-Sereshki, Coupland, Silverstone, Huang, Hegadoren, Carter and Malykhin2021; Ahmed-Leitao et al., Reference Ahmed-Leitao, Rosenstein, Marx, Young, Korte and Seedat2019). This research highlights the importance of future examinations of the development of specific hippocampal subfields and amygdala sub-nuclei in relation to maltreatment. Second, we averaged data for the left and right hemispheres, which may have led to us missing lateralized effects. Of note however, there is no consensus among findings pertaining to associations between adversity and brain structure about whether effects are lateralized (McLaughlin et al., Reference McLaughlin, Weissman and Bitrán2019; Rakesh & Whittle, Reference Rakesh and Whittle2021). Third, we inferred linear brain development trajectories from three time-points that were spaced relatively far apart. We did not investigate nonlinear patterns of brain change to prevent over-fitting, however, prior research does suggest nonlinear patterns of development for some brain regions investigated (e.g. Herting et al., Reference Herting, Johnson, Mills, Vijayakumar, Dennison, Liu and Tamnes2018). Further research is thus required to investigate maturational coupling of regions taking into account nonlinear developmental trajectories. Fourth, we did not conduct analyses pertaining to sexual abuse in our sample due to low endorsement. Given its prevalence in the general population (Barth, Bermetz, Heim, Trelle, & Tonia, Reference Barth, Bermetz, Heim, Trelle and Tonia2013), it is possible that a greater proportion of our sample had experienced sexual abuse but did not disclose it. Future research examining associations between maltreatment and structural brain maturation may benefit from investigating the validity and reliability of maltreatment assessment. Finally, we did not examine the effect of timing of exposure to maltreatment due to the lack of variability in this data in our sample (i.e. most participants endorsed lifetime maltreatment). The amygdala, hippocampus and PFC are suggested to be particularly sensitive to stress at different periods in development (Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009). The temporal window of exposure in our study was broad, including the whole childhood period. This may have masked any differential effects of maltreatment experienced at different ages. Future studies with larger sample sizes should explore the effects of timing of maltreatment exposure on neurodevelopment.
The present study provides novel insights into the relationship between maltreatment (and specifically neglect), and development of the amygdala and hippocampus and their maturational coupling with the PFC. Our findings suggest that maltreatment may have long-term effects on neurodevelopment, which may affect adolescent cognitive and socio-affective functioning. The significance of these findings for risk of mental ill-health are unclear, however. Nevertheless, the present study highlights the importance of the period of adolescence as a target for clinical intervention following the experience of childhood maltreatment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723001253.
Acknowledgements
This study was funded by the Colonial Foundation, the National Health and Medical Research Council (NHMRC; Australia; Program Grant 350241), and the Australian Research Council (ARC; Discovery Grants DP0878136 and DP109 2637).
No authors report any conflict of interest.









