Introduction
Psychosis continuum proposes psychotic experiences (PEs) and psychotic disorder exist on a continuum and have a shared etiology (van Os, Reference van Os2003; van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009). PEs are defined as infrequent hallucinations or delusions occurring in the absence of a psychotic disorder (Staines et al., Reference Staines, Healy, Coughlan, Clarke, Kelleher, Cotter and Cannon2022). The presence of PEs, particularly persistent and/or distressing PE, is associated with poorer functional and occupational outcomes, higher healthcare needs, and an elevated risk of developing a mental disorder (Bhavsar, McGuire, MacCabe, Oliver, & Fusar-Poli, Reference Bhavsar, McGuire, MacCabe, Oliver and Fusar-Poli2018; Healy et al., Reference Healy, Brannigan, Dooley, Coughlan, Clarke, Kelleher and Cannon2019; Jeppesen et al., Reference Jeppesen, Clemmensen, Munkholm, Rimvall, Rask, Jørgensen and Skovgaard2015; Rimvall et al., Reference Rimvall, van Os, Rask, Olsen, Skovgaard, Clemmensen and Jeppesen2020a, Reference Rimvall, van Os, Verhulst, Wolf, Larsen, Clemmensen and Jeppesen2020b, Reference Rimvall, Wolf, Olsen, Skovgaard, Clemmensen, Oxholm and Jeppesen2020c; Staines et al., Reference Staines, Healy, Kelleher, Cotter, Burns and Cannon2023a).
Prenatal and perinatal risk factors have been robustly shown to have a small-to-moderate effect on rates of psychotic disorder (Cannon, Jones, & Murray, Reference Cannon, Jones and Murray2002; Davies et al., Reference Davies, Segre, Estradé, Radua, De Micheli, Provenzani and Fusar-Poli2020). PE research has focused primarily on examining maternal behaviors such as smoking, alcohol consumption, and substance use. There is evidence that smoking during pregnancy is linked to increased risk for PEs (Barkhuizen, Taylor, Freeman, & Ronald, Reference Barkhuizen, Taylor, Freeman and Ronald2019; Betts, Williams, Najman, Scott, & Alati, Reference Betts, Williams, Najman, Scott and Alati2014; Dorrington et al., Reference Dorrington, Zammit, Asher, Evans, Heron and Lewis2014; Zammit et al., Reference Zammit, Thomas, Thompson, Horwood, Menezes, Gunnell and Harrison2009b). There is inconsistent evidence for alcohol consumption (Staines et al., Reference Staines, Healy, Coughlan, Clarke, Kelleher, Cotter and Cannon2022), with some papers finding it has an effect, and others not finding an association (Gregersen, Dreier, & Strandberg-Larsen, Reference Gregersen, Dreier and Strandberg-Larsen2020; Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a). The evidence of the association between maternal cannabis use and later PEs in children is more consistent, indicating maternal cannabis use increases risk of PEs in children (Bogdan, Sarah, & Alexander, Reference Bogdan, Sarah and Alexander2022; Bourque, Afzali, O'Leary-Barrett, & Conrod, Reference Bourque, Afzali, O'Leary-Barrett and Conrod2017; Fine et al., Reference Fine, Moreau, Karcher, Agrawal, Rogers, Barch and Bogdan2019; Paul et al., Reference Paul, Hatoum, Fine, Johnson, Hansen, Karcher and Bogdan2020).
Other pre/perinatal complications, such as medical complications, have been less frequently examined. Two previous cohort studies (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014; Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a) have measured other prenatal and perinatal complications as risk factors for PEs. Zammit et al. (Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a) found evidence that infection during pregnancy, any resuscitation after birth, and a low Apgar score, were all associated with an increased risk for PEs in childhood. Betts et al. (Reference Betts, Williams, Najman, Scott and Alati2014) found evidence that stressful life events during pregnancy increased risk of PEs in adulthood, but no evidence of any medical prenatal/perinatal complication. There is inconsistent evidence about the effects of birthweight, some studies finding that higher birthweight was protective (Thomas et al., Reference Thomas, Harrison, Zammit, Lewis, Horwood, Heron and Gunnell2009), and lower birthweight increased risk (Drakesmith et al., Reference Drakesmith, Dutt, Fonville, Zammit, Reichenberg, Evans and David2016), while others have found no effect of birthweight (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014).
One area that has not been investigated to date is the cumulative effect of prenatal and perinatal complications. Pre- and perinatal complications often co-occur (Bryson, Ioannou, Rulyak, & Critchlow, Reference Bryson, Ioannou, Rulyak and Critchlow2003; Östlund, Haglund, & Hanson, Reference Östlund, Haglund and Hanson2004; Yogev, Xenakis, & Langer, Reference Yogev, Xenakis and Langer2004), and the cumulative effect of prenatal complications have been shown to increase psychopathology in childhood (Roffman et al., Reference Roffman, Sipahi, Dowling, Hughes, Hopkinson, Lee and Dunn2021; Sood et al., Reference Sood, Delaney-Black, Covington, Nordstrom-Klee, Ager, Templin and Sokol2001). Additionally, previous studies (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014; Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a) have only examined the relationship between prenatal and perinatal complications with PEs at a single time-point. To our knowledge, no study has investigated whether these effects are developmentally specific or enduring. Similarly, no study has distinguished if these risk factors can differentiate those with multiple PE events (persistent), compared to one off PE events (transient).
The current study aims to answer three key questions:
(1) Are pre- and perinatal risk factors associated with frequency of PEs across late childhood (age 9–12)?
(2) Is there a cumulative effect of prenatal/perinatal risk factors on frequency of PEs?
(3) Are prenatal/perinatal risk factors differently related to transient and persistent PEs?
Methods
Participants
This study used data from the Adolescent Brain Cognitive Development (ABCD) study (abcdstudy.org), an ongoing prospective cohort study; full details are available elsewhere (Jernigan & Brown, Reference Jernigan and Brown2018; Karcher et al., Reference Karcher, Barch, Avenevoli, Savill, Huber, Simon and Loewy2018). Briefly, children were recruited at age 9–10 at baseline (n = 11 875) and followed up every 1–2 years. The ABCD sample was designed to mirror US population demographics by recruiting through geographically, demographically, and socioeconomically diverse school systems surrounding each of the research sites (Garavan et al., Reference Garavan, Bartsch, Conway, Decastro, Goldstein, Heeringa and Zahs2018), with the exception that ABCD at baseline oversampled for non-singleton births (Garavan et al., Reference Garavan, Bartsch, Conway, Decastro, Goldstein, Heeringa and Zahs2018). Exclusion criteria include non-fluency in English (child/parent), or if the child had contraindications to magnetic resonance imaging, extreme prematurity (<28 weeks gestation), history of neurological disorders, current diagnosis of schizophrenia, substance-use disorder, intellectual disability, or moderate/severe autism spectrum disorder. All measures used were from baseline (age 9–10), time-point 1 (age 10–11), and time-point 2 (age 11–12).
Within study sites, consenting parents and assenting children were primarily recruited through a probability sample of schools, summer camp programs, and community volunteers. The University of California at San Diego (San Diego, CA, USA) Institutional Review Board was responsible for the ethical oversight of the ABCD study. The secondary analysis of the data was approved by the Research Ethics Committee for the Royal College of Surgeons in Ireland.
Measures
Data were collected using in-person interviews. Parent report used the primary caregiver, including biological mothers (85%), biological fathers (10%), adoptive parents (2.5%), custodial parents (1%), and ‘other’ (1.5%).
Exposures
Prenatal and perinatal complications were collected using the primary caregiver report at baseline using the ABCD Developmental History Questionnaire (Kessler et al., Reference Kessler, Avenevoli, Costello, Green, Gruber, Heeringa and Zaslavsky2009; Merikangas, Avenevoli, Costello, Koretz, & Kessler, Reference Merikangas, Avenevoli, Costello, Koretz and Kessler2009). In line with previous research, these were scored dichotomously (absent/present) and analyzed collectively to measure cumulative risk (Roffman et al., Reference Roffman, Sipahi, Dowling, Hughes, Hopkinson, Lee and Dunn2021).
Prenatal complications
The following prenatal measures were included. These were collected at baseline from the primary caregiver, reported as dichotomous measure (Yes/No). The measures included were: severe nausea beyond 6th month of pregnancy, prenatal diabetes, high blood pressure, persistent proteinuria, urinary tract infection (UTI), pre-eclampsia/eclampsia/toxemia, severe anemia, rubella in the first 3 months of pregnancy, severe gallbladder attack, placental issues (previa, abruption, other), excessive bleeding requiring treatment, accident requiring medical attention, or other complication requiring medical attention. Each prenatal complication was examined separately, and the total number of prenatal complications was computed (0–12) for the prenatal complications cumulative score.
Perinatal complications
Perinatal complications were collected from the primary caregiver, reported as a binary response (Yes/No). The following prenatal measures were included, reported as dichotomous measures: being born jaundiced, requiring oxygen at birth, born blue, with a slow heartbeat, not breathing at first, rhesus incompatibility, a blood transfusion, or convulsions. Each perinatal complication was examined separately, and the total number of delivery complications was computed (0–8) for the cumulative perinatal complications score.
Fetal growth
In line with previous research (Class, Rickert, Larsson, Lichtenstein, & D'Onofrio, Reference Class, Rickert, Larsson, Lichtenstein and D'Onofrio2014; Dooley et al., Reference Dooley, Healy, Brannigan, Cotter, Clarke and Cannon2022) we opted to use fetal growth, rather than birthweight, by calculating the appropriateness of weight for their prenatal age. Measures of prenatal age (in weeks) and birth weight (kg) were parent reported at baseline. To calculate fetal growth, we used a linear regression to obtain the residuals from the association between prenatal age and birthweight. This allowed us to estimate the variance in birthweight, accounting for the child's prenatal age, giving us a proxy estimate of fetal growth.
Maternal behavior
Maternal smoking, alcohol consumption, and substance use was collected from the Developmental History Questionnaire (Kessler et al., Reference Kessler, Avenevoli, Costello, Green, Gruber, Heeringa and Zaslavsky2009; Merikangas et al., Reference Merikangas, Avenevoli, Costello, Koretz and Kessler2009) and was defined as engaging in those activities at any point during pregnancy. Maternal smoking was collected from question ‘tobacco? How many times a day?’, treated as a binary if they smoked during pregnancy, regardless of frequency. Alcohol consumption was measured as ‘Alcohol? Average drinks per week?’, treated as a binary if they drank during pregnancy, regardless of frequency. Substance use was created by combining questions of ‘Marijuana? How many times per day?/Cocaine/Crack? How many times per day?/Heroin/Morphine? How many times per day?/Oxycontin? How many times per day?’. A binary ‘yes/no’ variable was created for substance use, based on a positive answer to any of the substance use questions, regardless of frequency.
Covariates
A demographic questionnaire measured child ethnicity and sex, and parental income. Ethnicity was measured as a categorical variable (Black, White, Asian, Hispanic, other), and sex (male/female), both reported at baseline. Income level was calculated as a categorical variable of combined (if two-parent household) income (<$5k, $5–11k, $12k–15 999, $16k–24,999, $25k–34 999, $35k–49 999, $50k–74 999, $75k–99 999, $100k–199 999, $200k+). Maternal age at time of birth was reported by the mother at baseline.
Family history of mental illness was collected using the parent-reported Family History Assessment (Rice et al., Reference Rice, Reich, Bucholz, Neuman, Fishman, Rochberg and Begleiter1995). We calculated the number of family members with any mental illness (alcohol addiction, drug addiction, conduct disorder, depression, bipolar, anxiety, psychosis, suicide attempt/death by suicide, hospitalization for mental illness, use of clinical services). A second measure examining specifically the number of relatives with psychosis was also calculated.
Outcomes
Psychotic experiences
PEs were measured using the self-report Prodromal Questionnaire-Brief, Child version (PQ-BC) (Karcher et al., Reference Karcher, Barch, Avenevoli, Savill, Huber, Simon and Loewy2018). This is adapted from the adult version (Loewy, Pearson, Vinogradov, Bearden, & Cannon, Reference Loewy, Pearson, Vinogradov, Bearden and Cannon2011), and similarly measures 21 items on a range of positive psychotic symptoms. For each symptom the participant endorsed, they were asked whether the symptom ‘bothered them (Yes/No)’. Those who affirmatively reported that the PE was distressing were asked to score level of distress (1–5).
PE was measured as frequency of distressing PE, defined as a participant scoring distress about PE as ⩾3, at each time-point respectively. In the PQ-BC, distress is rated on a 5 point scale, using appropriate age visuals to help children assess distress; for full details see Karcher et al. (Reference Karcher, Barch, Avenevoli, Savill, Huber, Simon and Loewy2018). The cut-off of ⩾3 is in line with previous research (Karcher et al., Reference Karcher, Paul, Johnson, Hatoum, Baranger, Agrawal and Bogdan2022). Inclusion of this cut-off was used to ensure that an actual PE is being reported, utilizing previous studies which identify distress as a marker of a significant PE in children (Rimvall et al., Reference Rimvall, van Os, Rask, Olsen, Skovgaard, Clemmensen and Jeppesen2020a, Reference Rimvall, Wolf, Olsen, Skovgaard, Clemmensen, Oxholm and Jeppesen2020c).
Persistent v. transient PEs
Persistent was defined as at least one distressing PE at more than one time (McGrath et al., Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet, Bruffaerts and Kessler2015). Transience was defined as a distressing PE at only one time-point. Control was defined as reporting no distressing PE at any time-point. Only those with data at all 3 time-points were included for this analysis.
Analysis
Mixed models were used for each model; the random effects allowed us to capture variance across research sites and families. In all models, continuous variables were standardized. To allow for standardization, income was treated as a continuous variable. To examine if the risk factors were independently associated with distressing childhood PEs, the model was adjusted for a number of known risk factors for PEs. The fully adjusted model accounted for demographic and sample differences in sex, ethnicity, over-sampling of twins/triplets, maternal age, socioeconomic status, family history of psychosis/mental illness, and maternal behaviors (smoking, alcohol consumption, and substance use).
For the analysis of individual prenatal/perinatal risk factors, and for the cumulative risk of pre-/peri-natal risk factors, Gamma distribution linear mixed-effects models were used. The main effect, and the interaction between the exposure (prenatal/perinatal complication) and time are also reported. This allowed us to assess whether the effects of exposures remitted as the participants got older, or remained consistent.
To account for multiple comparisons for the individual prenatal/perinatal risk factors the Benjamini and Hochberg's (Reference Benjamini and Hochberg1995) false-discovery date correction for multiple comparisons was used. This was used for the main effect and interactions, respectively.
Each maternal behavior was also examined as a prenatal risk factor. For these models, all measures expect maternal behaviors were included as covariates. To account for multiple comparisons the Benjamini and Hochberg's (Reference Benjamini and Hochberg1995) false-discovery date correction for multiple comparisons was also used. Examining differences between transient and persistent PEs, binomial distribution regression logistic mixed-effects models were used.
Participants without the outcomes, exposures, or confounders were excluded from the respective analyses where data were missing. The included sample, for each model, is reported in each table.
Results
Descriptive
At baseline, 11 872 completed the PQ-BC; those who had not completed baseline (n = 4) were excluded from analysis. At follow-up T1, 651 did not complete the measure (n = 11 221), and at follow-up T2 1462 did not complete the measure (n = 10 410). We found small-to-moderate correlations between prenatal and perinatal complications (online Supplementary eFigures 1 and 2). Children who reported PE at baseline were compared to controls (Table 1).
Table 1. Differences in demographic, income, familial history, maternal risk behaviors, prenatal complications, perinatal complications, and fetal growth between children with PEs and controls, at baseline
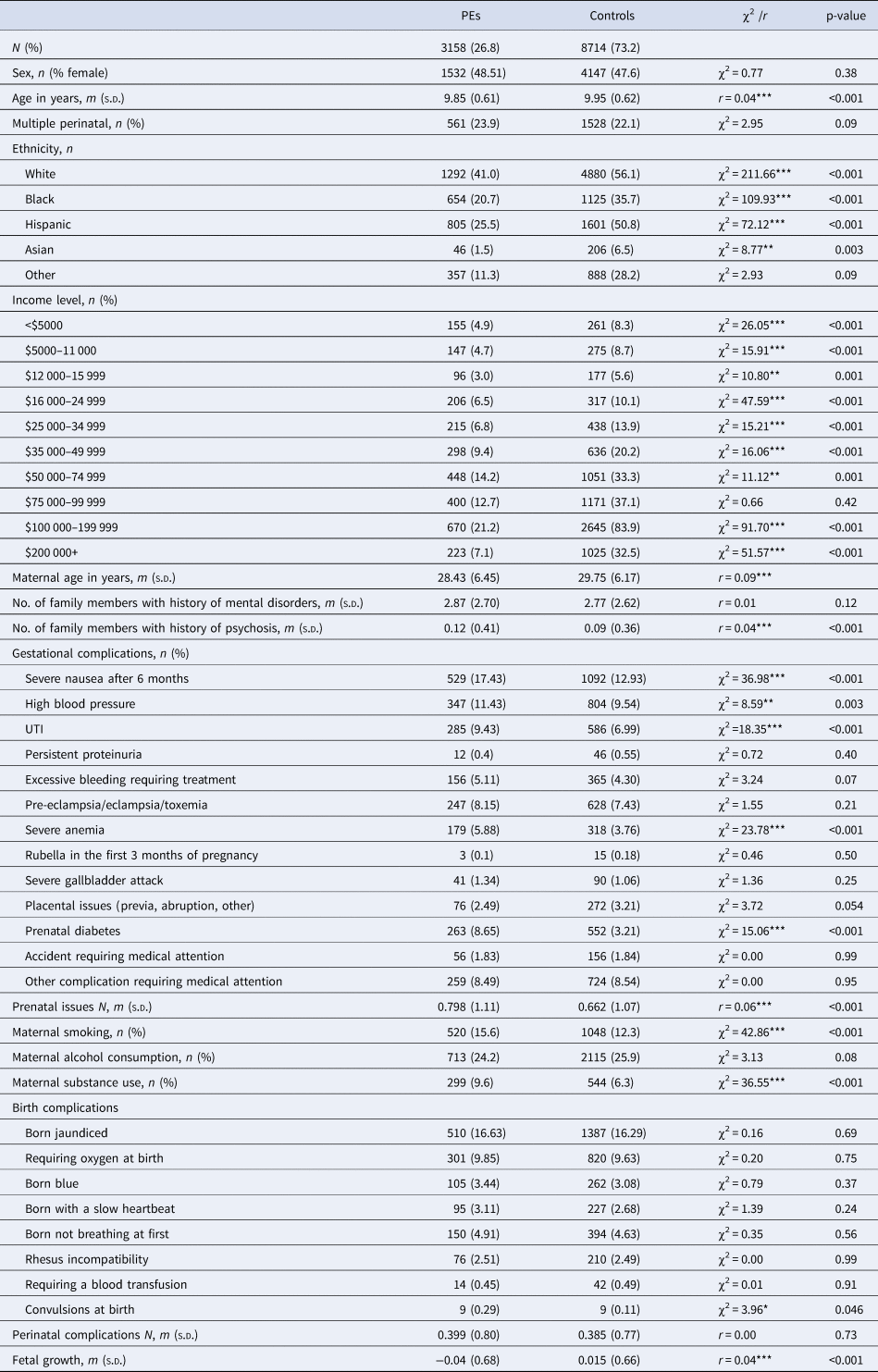
PE, psychotic experience (distressing); χ2, chi-squared test; r, effect size of Wilcoxon test.
***p < 0.001, **p< 0.01, *p < 0.05.
Children whose parents reported their ethnicity as Black, Hispanic, or Asian were proportionally more likely to report PE than those identified as White (Table 1). Income level showed a trend of more people having PEs being in the lower income brackets, and less people with PE in the higher income brackets (Table 1). Maternal age was significantly younger for those with PE compared to controls (Table 1). Number of family members with mental disorder/psychosis was also higher for PE compared to controls (Table 1).
Considering group-level differences of the main exposures, maternal smoking and substance use were higher in those with PE than controls at baseline. Several prenatal medical complications and one perinatal complication were more common in those with PE at baseline (Table 1). The number of prenatal complications, but not perinatal complications, were higher in those with PE at baseline (Table 1). Fetal growth was on average below average in the PE group, while it was above average in the control group (Table 1).
Any prenatal/perinatal measure reported by less than 100 people with PEs was excluded from the main analysis. Prenatal complications excluded on this criteria included persistent proteinuria, rubella in the first 3 months of pregnancy, a severe gallbladder attack, placental issues, or accident requiring medical attention (Table 1). Perinatal complications excluded were blood transfusions or convulsions (Table 1).
Prenatal and perinatal risk factors for PE
Two included individual measures of prenatal complication measures (UTI and severe anemia) were associated with an increase in frequency of PEs in childhood, which survived correction for multiple comparisons (Table 2). Maternal smoking had a significant main effect (Table 2) when correcting for multiple comparisons, showing maternal smoking increases the frequency of distressing PEs in children. As children entered adolescence the main effect of maternal smoking was moderately attenuated (Table 2).
Table 2. Prenatal risk factors on rates of PEs in childhood, accounting for demographic, economic, familial history, and maternal risk behaviors
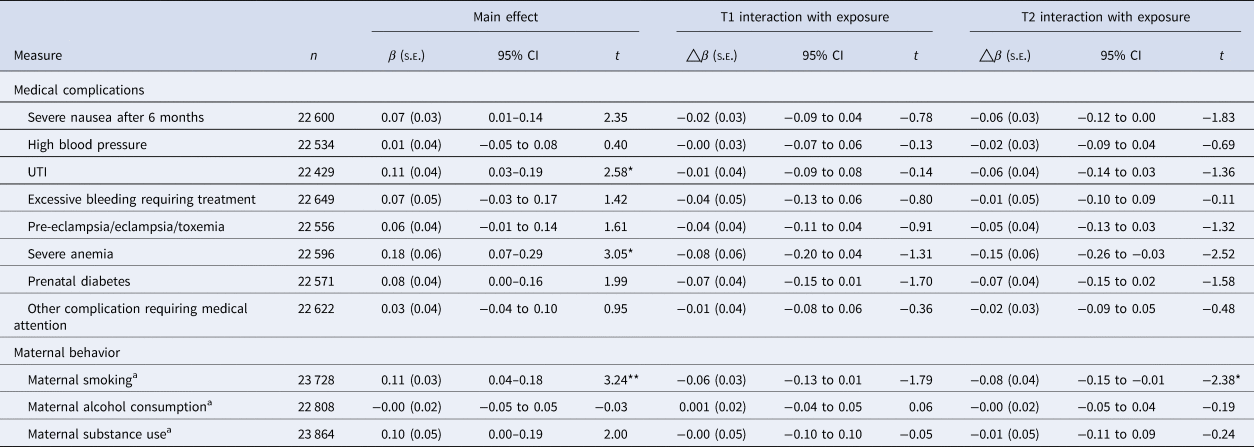
PE, distressing psychotic experiences; β, estimate; △β=, change in estimate; t, t-value.
p-Value is corrected for multiple comparisons, using Benjamini and Hochberg's (Reference Benjamini and Hochberg1995) false-discovery rate correction.
a Maternal smoking, alcohol consumption, and substance use did not do the fully adjusted model, only controlling for minimally adjusted model, family income, family history of psychosis, and mental disorders; model fully adjusted; accounting for demographic (sex, multiple births, ethnicity, maternal age and random effect of site, and family ID), family income, family history of psychosis and mental disorder, maternal risk behaviors in pregnancy (maternal alcohol consumption, smoking, substance use).
**p < 0.01, *p < 0.05.
Fetal growth was not associated with frequency of PEs in childhood (Table 3). No perinatal medical complication was associated with an elevated risk for PE in childhood (Table 3).
Table 3. Perinatal risk factors on rates of PEs in childhood, accounting for demographic, economic, familial history, and maternal risk behaviors
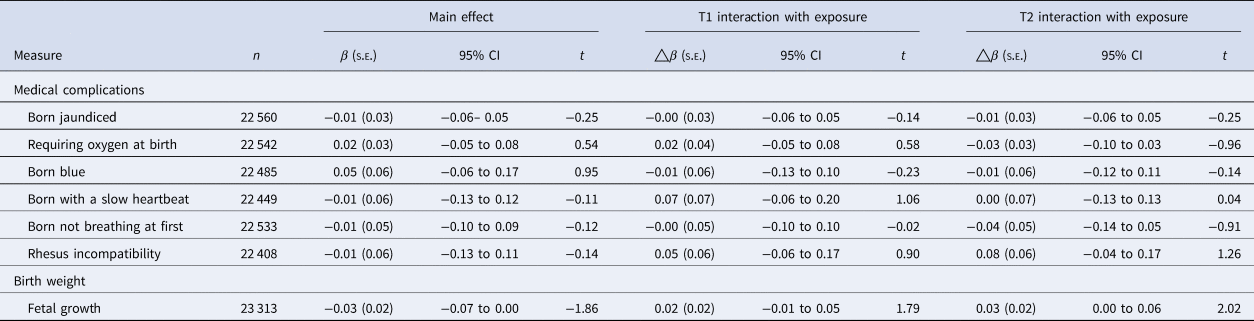
PE, distressing psychotic experiences.
Model fully adjusted; accounting for demographic (sex, multiple births, ethnicity, maternal age and random effect of site, and family ID), family income, family history of psychosis and mental disorder, maternal risk behaviors in pregnancy (maternal alcohol consumption, smoking, substance use).
p-value is corrected for multiple comparisons, using Benjamini and Hochberg's (Reference Benjamini and Hochberg1995) false-discovery rate correction.
**p < 0.01, *p < 0.05.
Cumulative risk of prenatal/perinatal risk factors and PE
Cumulative risk of prenatal complications (median = 0; interquartile range [IQR] = 1; range(0–13)), and perinatal complications (median = 0; IQR = 1; range(0–8)) was low. Controlling for covariates, every additional prenatal complication was associated with a small increase in frequency of PEs (β = 0.03; 95% confidence interval [CI] 0.01–0.05, t = 3.12, p = 0.002). This effect showed a non-significant reduction at time-point 1 (β = −0.01; 95% CI −0.03 to 0.01, t = −1.00, p = 0.32), and moderately attenuated by time-point 2, i.e. as the child enters adolescence (β = −0.02; 95% CI −0.04 to −0.00], t = −2.00, p = 0.05) (Fig. 1). The cumulative risk of perinatal risk factors was non-significant (β = 0.00; 95% CI −0.03 to 0.01], t = 0.29, p = 0.82).
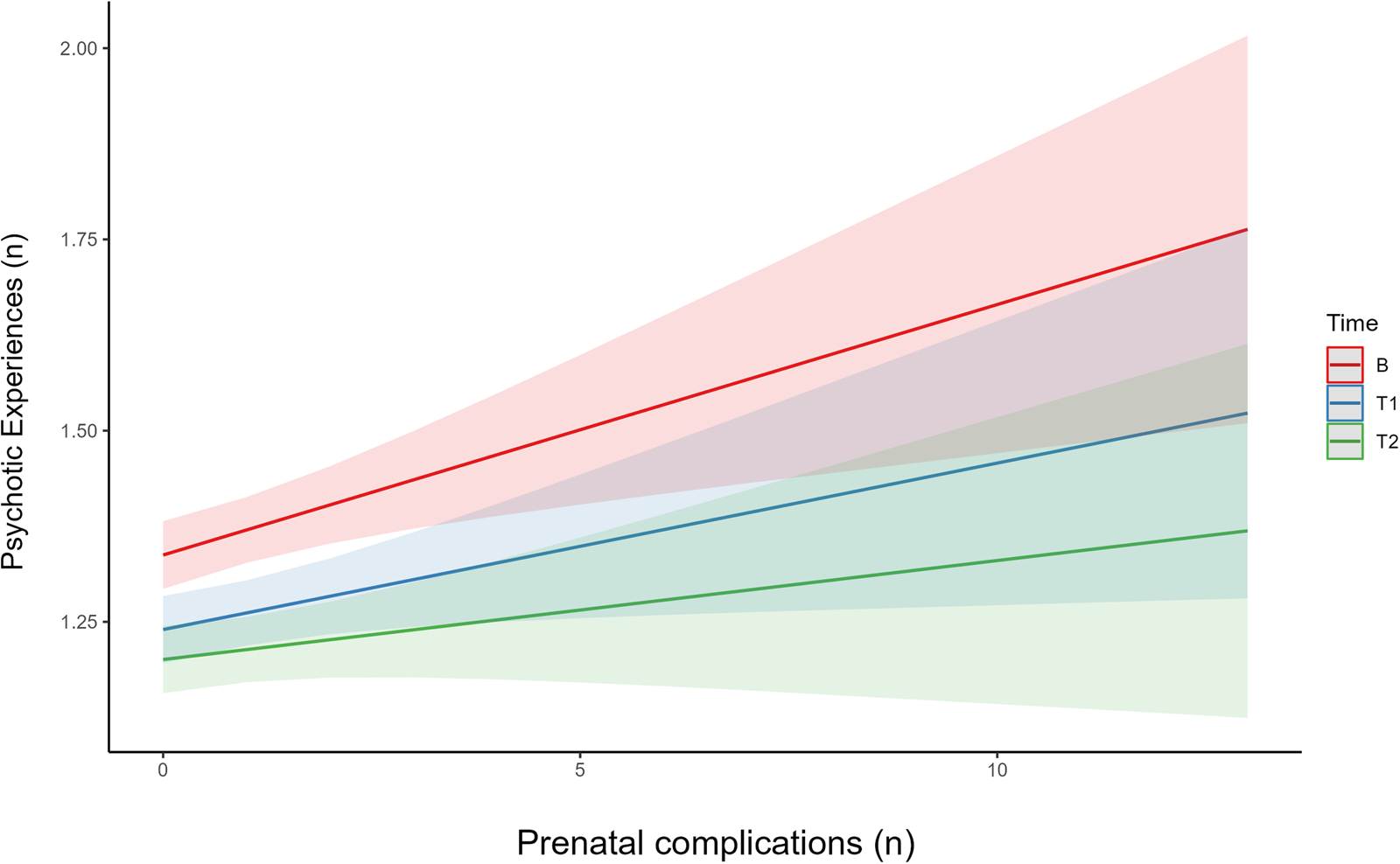
Figure 1. Interaction effects between number of prenatal complications on number of PE, divided by time-point. The interaction graph allows prediction of the effect of number of prenatal complications on rates of PE, split into the three time-points (baseline, follow-up 1, and 2). This graph also allows us to examine if there in a difference in effect at different time-points, based on the slope of the line. This graph indicates that while there is an effect of number of prenatal complications on rate of PE, it declines at later time-points i.e. as the children get older. B, baseline time-point, T1, follow-up 1 (1 year), T2, follow up 2 (2 years).
Persistent v. transient PEs
Only those with measures of PEs at all three time-points were included for analysis (n = 10 182). Participants were grouped into control (n = 6261), transient (n = 2236), and persistent (n = 1685).
Persistent and transient PE groups had a significantly higher number of prenatal complications in the fully adjusted model, compared to the control group (Table 4). For each prenatal complication, participants were 8% more likely to be in the persistent or transient group, compared to controls (Table 4). There were no significant differences in prenatal complications between the persistent PE and transient PE groups. The number of perinatal complications and average fetal growth were not associated with group membership (Table 4).
Table 4. Effects of prenatal complications, perinatal complications, fetal growth on risk of PEs, comparing between different PE groups (control, transient, persistent)
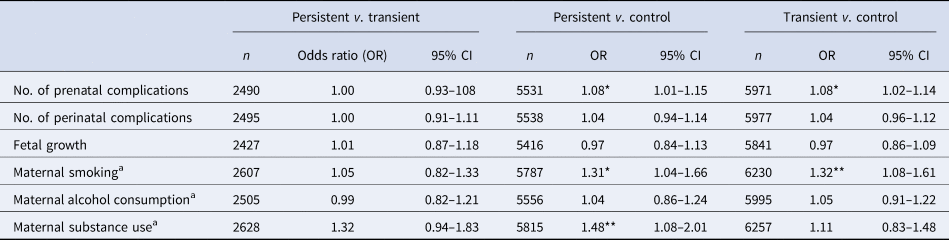
Persistent, reported distressing PE at 2–3 time-points; transient, reported distressing PE at only 1 time-point; control, reported distressing PE at no time-points; β, estimate; E, standard error; t, t-value.
a Maternal smoking, alcohol consumption, and substance use did not do the fully adjusted model, only controlling for minimally adjusted model, family income, family history of psychosis and mental disorders. Model 1: accounting for demographic (sex, multiple births, ethnicity, maternal age and random effect of site, and family ID). Model 2: accounting for family income, family history of psychosis and mental disorder, maternal risk behaviors in pregnancy (maternal alcohol consumption, smoking, substance use) + model 1.
***p < 0.001, **p < 0.01, *p < 0.05.
In the fully adjusted model, maternal smoking was a group differentiator for persistent and transient PEs compared to control, representing a 31% and 32% risk respectively (Table 4). Maternal substance use was a differentiator for persistent PE compared to control, representing an elevated risk of being in the persistent PE group by 48% (Table 4). No measure of maternal behavior was a differentiator between the transient and persistent PE groups (Table 4).
Discussion
This study examined prenatal and perinatal factors as risk factors for frequency of distressing childhood PE and persistent PE, and found several risk factors; UTIs, severe anemia, cumulative risk of prenatal medication complication, maternal smoking, and maternal substance use.
Previous research has shown that a UTI during pregnancy is associated with preterm labor (Balachandran et al., Reference Balachandran, Jacob, Al Awadhi, Yahya, Catroon, Soundararajan and Hussein2022), susceptibility to pediatric infections (Cohen, Gutvirtz, Wainstock, & Sheiner, Reference Cohen, Gutvirtz, Wainstock and Sheiner2019), and symptoms of attention deficit and hyperactivity disorder (ADHD) (Mann & McDermott, Reference Mann and McDermott2011). Our finding adds to this literature by showing the presence of maternal UTI increases the frequency of distressing PE across childhood. Similarly, severe anemia has previously been linked to higher rates of perinatal complications (Shi et al., Reference Shi, Chen, Wang, Sun, Guo, Ma and Qiao2022) and neurodevelopmental difficulties in offspring, including ADHD and autism spectrum disorder (Wiegersma, Dalman, Lee, Karlsson, & Gardner, Reference Wiegersma, Dalman, Lee, Karlsson and Gardner2019).
One explanation for why prenatal complications could increase risk for subsequent PEs is the role of inflammation. Inflammation is a natural biological response to infection or physical trauma (Scott, Khan, Cook, & Duronio, Reference Scott, Khan, Cook and Duronio2004). Evidence suggests that inflammation (acute and chronic) is associated with psychotic phenomena (Föcking et al., Reference Föcking, Sabherwal, Cates, Scaife, Dicker, Hryniewiecka and Cotter2019; Mongan et al., Reference Mongan, Föcking, Healy, Susai, Heurich and Wynne2021; Mongan, Ramesar, Föcking, Cannon, & Cotter, Reference Mongan, Ramesar, Föcking, Cannon and Cotter2020). It has been hypothesized that aberrant inflammatory processes may be the explanation for this process (Mongan et al., Reference Mongan, Ramesar, Föcking, Cannon and Cotter2020). Within this context, maternal infection may be the mechanism by which aberrant inflammatory processes develop, through abnormal immune responses in utero (Anderson et al., Reference Anderson, Hoath, Zammit, Meyer, Pell, Mackay and Smith2016; Khandaker, Zimbron, Lewis, & Jones, Reference Khandaker, Zimbron, Lewis and Jones2013). Previous studies which have examined maternal acute inflammation during pregnancy but did not find an association with PEs (Anderson et al., Reference Anderson, Hoath, Zammit, Meyer, Pell, Mackay and Smith2016; Ramsay et al., Reference Ramsay, Surcel, Björnholm, Kerkelä, Khandaker and Veijola2021). Chronic inflammation is a more significant inflammatory marker for subsequent psychotic symptoms (Byrne et al., Reference Byrne, Healy, Mongan, Susai, Zammit, Föcking and Cotter2022; Rasmussen et al., Reference Rasmussen, Ladelund, Haupt, Ellekilde, Poulsen, Iversen and Andersen2016). Prenatal complications in this study may be experienced as chronic/long lasting e.g. UTI. Therefore, the finding of this study may support the role of chronic inflammation. However, to determine the mechanisms behind the finding, additional research using biological data is required.
Maternal smoking was a risk factor for frequency and persistent distressing PE in this study. Previous research in PE has observed maternal smoking as a risk factor for PE (Barkhuizen et al., Reference Barkhuizen, Taylor, Freeman and Ronald2019; Dorrington et al., Reference Dorrington, Zammit, Asher, Evans, Heron and Lewis2014; Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a). As was observed in this study, maternal smoking as a risk factor declined in adolescence. Maternal substance use was also found to be a risk factor for PE. Previous research has primarily focused on maternal cannabis consumption, with consistent evidence showing it increases rates of PE (Bogdan et al., Reference Bogdan, Sarah and Alexander2022; Bolhuis et al., Reference Bolhuis, Kushner, Yalniz, Hillegers, Jaddoe, Tiemeier and El Marroun2018; Fine et al., Reference Fine, Moreau, Karcher, Agrawal, Rogers, Barch and Bogdan2019; Paul et al., Reference Paul, Hatoum, Fine, Johnson, Hansen, Karcher and Bogdan2020; Staines et al., Reference Staines, Healy, Murphy, Byrne, Murphy, Kelleher and Cannon2023b). Explanations for the association between substance use and mental disorders have been suggested to be due to a shared etiology with addictive behaviors (Myles et al., Reference Myles, Newall, Curtis, Nielssen, Shiers and Large2012). Similarly, addiction shows shared environmental risk factors to mental disorders and PE e.g. socioeconomic status (Patrick, Wightman, Schoeni, & Schulenberg, Reference Patrick, Wightman, Schoeni and Schulenberg2012; Staines et al., Reference Staines, Healy, Coughlan, Clarke, Kelleher, Cotter and Cannon2022; Werner, Malaspina, & Rabinowitz, Reference Werner, Malaspina and Rabinowitz2007). It is therefore possible that the associations found in this study may be driven by shared genetic and/or environmental factors between the pregnant person and infant.
Second, smoking has been associated with chronic inflammation (Lee, Taneja, & Vassallo, Reference Lee, Taneja and Vassallo2012), and it is possible in utero exposure to smoking might also be explained by the same mechanism discussed above. One consideration is that cannabis consumption is often combined with tobacco use (Weinberger et al., Reference Weinberger, Delnevo, Wyka, Gbedemah, Lee, Copeland and Goodwin2019). In the ABCD study, of the participants exposed to cannabis usage during pregnancy (n = 655), 44% (n = 289) were also exposed to tobacco use during pregnancy (Paul et al., Reference Paul, Hatoum, Fine, Johnson, Hansen, Karcher and Bogdan2020). Therefore, it should not be ruled out that some of the effects observed in this study may have been driven in part by those who smoked tobacco and cannabis in combination. However, this group only represents 18%, of all those who smoked tobacco while pregnant (Table 1). Overall this study suggests that tobacco and substance use represent an independent risk for distressing childhood PEs.
Perinatal complications did not show a significant effect in this study, differing from previous research on PEs (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014; Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a). One consideration is that prenatal complications in this study are experienced over extended periods of time or chronically, while exposure to perinatal complications is becoming shorter in newer cohorts. Evidence shows that countries with substantial health care services have lower rates of birth asphyxia and hypoxia (Mukhtar-Yola et al., Reference Mukhtar-Yola, Audu, Olaniyan, Akinbi, Dawodu and Donovan2018; World Health Organization, 2004). Previous cohorts on this topic were born in 1981 (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014) and 1991–1992 (Zammit et al., Reference Zammit, Odd, Horwood, Thompson, Thomas, Menezes and Harrison2009a), in comparison to the ABCD sample born in 2008 (Garavan et al., Reference Garavan, Bartsch, Conway, Decastro, Goldstein, Heeringa and Zahs2018).
Finally, lower than average fetal growth in this study was not found to be a risk factor for PEs, or higher than average fetal growth as a protective factor. Previous studies (Cannon et al., Reference Cannon, Jones and Murray2002; Davies et al., Reference Davies, Segre, Estradé, Radua, De Micheli, Provenzani and Fusar-Poli2020) have observed an association between lower birth weight and psychotic disorder. The PE literature has been less conclusive, with mixed evidence for birthweight (Betts et al., Reference Betts, Williams, Najman, Scott and Alati2014; Drakesmith et al., Reference Drakesmith, Dutt, Fonville, Zammit, Reichenberg, Evans and David2016) and some evidence for birthweight as a protective factor (Thomas et al., Reference Thomas, Harrison, Zammit, Lewis, Horwood, Heron and Gunnell2009).
Limitations
First, all pre- and perinatal information was reported retrospectively by the primary caregiver 9–10 years post-partum. Therefore, it cannot be assumed that all medical complications or maternal behaviors were reported accurately. Similarly, 95% of the records were collected from the biological parents (85% mother, 10% father). This means that 15% of the total ABCD sample were not reported by the individuals who were pregnant, so risk of missing data or recall bias may be more likely in this subgroup. Second, there are some limitations to the generalizability of the ABCD sample used, specifically with the over representation of participants in the higher income bracket ($100 000–200 000). This could be a sample with better access to healthcare than the general population. Additionally, current diagnosis of schizophrenia or substance use in the child was an exclusion criterion, meaning it may not represent the most severe end of the psychosis spectrum. PEs were self-report from relatively young participants, whose interpretation of the question is unknown (age 9–10 baseline, 11–12 time-point 2). Distress level was included in PE ratings in an attempt to distinguish strong perceptual experiences from common everyday occurrences. Finally, this study controlled for a significant number of known covariates, to consider if the prenatal/perinatal risk factors were independent risk factors for childhood distressing PEs. This was an advantage of the large sample size, and addressed limitations of previous studies. However, several covariates e.g. family history of mental disorders, can also increase risk for prenatal complications (Sūdžiūtė et al., Reference Sūdžiūtė, Murauskienė, Jarienė, Jaras, Minkauskienė, Adomaitienė and Nedzelskienė2020), as well as being a risk factor for PEs. Therefore, in considering prenatal complications, it is important to be cognizant that these risk factors may not independently occur, and so improving prenatal care is only part of reducing these risk factors.
Conclusions
Prenatal and perinatal complications have been described as the ‘canaries in the coalmine’ (Cannon, Healy, Clarke, & Cotter, Reference Cannon, Healy, Clarke and Cotter2020) for psychosis, and this research supports that description. Most substantially, maternal behaviors (smoking, substance use) showed the largest effect, and highlight the continued need for education and promotion of healthy behaviors during pregnancy, and support for treatable complications of pregnancy, such as severe anemia. The interaction between time and prenatal complications suggests that these may be predominantly risk factors for childhood PEs. However, PEs in childhood can have long-term adverse outcomes (Rimvall et al., Reference Rimvall, van Os, Rask, Olsen, Skovgaard, Clemmensen and Jeppesen2020a, Reference Rimvall, van Os, Verhulst, Wolf, Larsen, Clemmensen and Jeppesen2020b). Therefore, prevention of maternal infection should be a priority, not just for the health of the pregnant person, but also for the future health of the fetus.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724000187.
Funding statement
Dr Staines and Prof. Cannon are funded by the European Research Council Consolidator Award (Grant Code: 724809 iHEAR). Dr Staines and Prof. Cotter are funded by the Health Research Board project PSI-STAR (Grant Code: 21090A01). Prof. Cotter is funded by a Wellcome Trust Innovations Award, number 220438Z/20/Z, in part by a research grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3948415, and co-funded under the European Regional Development Fund and by FutureNeuro industry partners.
Competing interests
None.
Ethical standards
Consent was obtained from the parents of the participants, and active assent was obtained from the children. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








