Neurocognitive dysfunction is common in psychiatric disorders including schizophrenia (Reichenberg, Reference Reichenberg2010; Saykin et al., Reference Saykin, Gur, Gur, Mozley, Mozley, Resnick and Stafiniak1991), posttraumatic stress (Aupperle, Melrose, Stein, & Paulus, Reference Aupperle, Melrose, Stein and Paulus2012; Scott et al., Reference Scott, Matt, Wrocklage, Crnich, Jordan, Southwick and Schweinsburg2015), and mood disorders (Bredemeier, Warren, Berenbaum, Miller, & Heller, Reference Bredemeier, Warren, Berenbaum, Miller and Heller2016; Merikangas et al., Reference Merikangas, Cui, Calkins, Moore, Gur, Gur and Merikangas2017). It is prevalent across diagnoses (Abramovitch, Short, & Schweiger, Reference Abramovitch, Short and Schweiger2021; Weiser et al., Reference Weiser, Reichenberg, Rabinowitz, Knobler, Lubin, Yazvitzky and Davidson2004) and in those scoring high on internalizing symptoms dimensions (Chavez-Baldini et al., Reference Chavez-Baldini, Nieman, Keestra, Lok, Mocking, de Koning and Denys2023; Zhu et al., Reference Zhu, Womer, Leng, Chang, Yin, Wei and Wang2019). Because neurocognitive deficits often precede the onset of symptoms (Dickson, Laurens, Cullen, & Hodgins, Reference Dickson, Laurens, Cullen and Hodgins2012; Gur et al., Reference Gur, Calkins, Satterthwaite, Ruparel, Bilker, Moore and Gur2014), they may indicate risk for psychopathology (Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014; Jonas et al., Reference Jonas, Lian, Callahan, Ruggero, Clouston, Reichenberg and Kotov2022; Moffitt et al., Reference Moffitt, Arseneault, Belsky, Dickson, Hancox, Harrington and Caspi2011; Rotstein, Fund, Levine, Reichenberg, & Goldenberg, Reference Rotstein, Fund, Levine, Reichenberg and Goldenberg2023) and elucidate neurodevelopment and psychopathology.
Most studies report impaired cognitive functioning in those with mental illness or high levels of a symptom dimension (East-Richard, R. -Mercier, Nadeau, & Cellard, Reference East-Richard, R. -Mercier, Nadeau and Cellard2020; Weiser et al., Reference Weiser, Reichenberg, Rabinowitz, Knobler, Lubin, Yazvitzky and Davidson2004), yet results for distinct neurocognitive profiles associated with specific dimensions of psychopathology vary. This heterogeneity may relate to diverse approaches in transdiagnostic studies (Astle, Holmes, Kievit, & Gathercole, Reference Astle, Holmes, Kievit and Gathercole2022). One approach has compared cognitive functioning across traditional categorical diagnoses with shared characteristics. For example, studies have examined generalized and disorder-specific executive functioning across multiple disorders with social impairment, including social anxiety, autism, and early psychosis (Demetriou et al., Reference Demetriou, Song, Park, Pepper, Naismith, Hermens and Guastella2018). Others have examined differential and shared patterns of neurocognitive performance across mood disorders (Merikangas et al., Reference Merikangas, Cui, Calkins, Moore, Gur, Gur and Merikangas2017). Individuals with bipolar I disorder performed more accurately in complex cognition compared to controls, while individuals with major depressive disorder performed more accurately in emotion recognition, and patients across disorders showed similar speed performance across domains. While transdiagnostic, such findings still rest on categorical classification of the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) and reflect the shortcomings of these categories, including variability within diagnoses, comorbidity, and exclusion of sub-clinical populations with arbitrary thresholds (Astle et al., Reference Astle, Holmes, Kievit and Gathercole2022).
An alternative to categorical approaches is dimensional models of psychopathology, such as NIMH's Research Domain Criteria (RDoC; Insel, Reference Insel2014) and the Hierarchical Taxonomy of Psychopathology (HiTOP; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017) frameworks. Mental disorders are conceptualized as reflecting interactions among continuous parameters, with interacting symptom dimensions or factors (Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014). These models often incorporate a general psychopathology factor accounting for shared variance across dimensions (Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014; Lahey et al., Reference Lahey, Applegate, Hakes, Zald, Hariri and Rathouz2012; Lahey, Krueger, Rathouz, Waldman, & Zald, Reference Lahey, Krueger, Rathouz, Waldman and Zald2017), and specific factors reflecting distinct psychopathology dimensions. Dimensional models offer one method to model psychiatric comorbidity, and the structure of these models has been examined (Kim & Eaton, Reference Kim and Eaton2015; Michelini et al., Reference Michelini, Barch, Tian, Watson, Klein and Kotov2019; Ringwald, Forbes, & Wright, Reference Ringwald, Forbes and Wright2023; Sunderland et al., Reference Sunderland, Forbes, Mewton, Baillie, Carragher, Lynch and Slade2021). One promising approach is bifactor models (Moore et al., Reference Moore, Kaczkurkin, Durham, Jeong, McDowell, Dupont and Lahey2020), which specify a general factor of psychopathology and orthogonal individual factors, where scale items are allowed to load on both the general psychopathology factor and one specific factor (Reise, Moore, & Haviland, Reference Reise, Moore and Haviland2010). Thus, bifactor models can assess dimensionality of psychopathology by isolating the shared variance explained by general psychopathology from the specific variance explained by individual symptom dimensions (Reise, Reference Reise2012).
Despite extensive research on neurocognitive functioning in mental health disorders, few studies have examined associations among broad sets of cognitive domains and psychopathology symptom dimensions. Studies of transdiagnostic samples focused on associations with single cognitive domains: executive functioning (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018; Demetriou et al., Reference Demetriou, Park, Pepper, Naismith, Song, Thomas and Guastella2021, Reference Demetriou, Song, Park, Pepper, Naismith, Hermens and Guastella2018; Romer & Pizzagalli, Reference Romer and Pizzagalli2021; Shanmugan et al., Reference Shanmugan, Wolf, Calkins, Moore, Ruparel, Hopson and Satterthwaite2016; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017), processing speed (Kramer, Willcutt, Peterson, Pennington, & McGrath, Reference Kramer, Willcutt, Peterson, Pennington and McGrath2023), or social cognition (Gur, Moore, Calkins, Ruparel, & Gur, Reference Gur, Moore, Calkins, Ruparel and Gur2017). Other studies have evaluated a broad range of cognitive domains and dimensional psychopathology but used DSM-based categorical diagnoses as inclusion criteria (Chavez-Baldini et al., Reference Chavez-Baldini, Nieman, Keestra, Lok, Mocking, de Koning and Denys2023; Merikangas et al., Reference Merikangas, Cui, Calkins, Moore, Gur, Gur and Merikangas2017; Service et al., Reference Service, Vargas Upegui, Castaño Ramírez, Port, Moore, Munoz Umanes and Freimer2020; Zhu et al., Reference Zhu, Womer, Leng, Chang, Yin, Wei and Wang2019). Other transdiagnostic research has examined cognitive functioning across limited psychopathology dimensions, such as internalizing, externalizing, or attention deficit symptomatology (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018; Brislin et al., Reference Brislin, Martz, Joshi, Duval, Gard, Clark and Sripada2022). Most studies in youth have focused on associations between psychopathology and global cognition or executive function (Demetriou et al., Reference Demetriou, Park, Pepper, Naismith, Song, Thomas and Guastella2021; Kramer et al., Reference Kramer, Willcutt, Peterson, Pennington and McGrath2023; Romer & Pizzagalli, Reference Romer and Pizzagalli2021; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017), limiting the ability to identify neurocognitive correlates of psychopathology phenotypes. A better understanding is needed regarding the nature of neurocognitive deficits, both shared and unique, across psychopathology dimensions.
The present study aimed to evaluate relationships among cognitive functioning, general psychopathology, and distinct dimensions of psychopathology in a large sample of children, adolescents, and young adults. We utilized data from the community-based Philadelphia Neurodevelopmental Cohort (PNC), which included a structured psychopathology interview and a neurocognitive assessment across domains (Calkins et al., Reference Calkins, Merikangas, Moore, Burstein, Behr, Satterthwaite and Gur2015; Gur et al., Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012). Prior PNC studies examined links between psychopathology and cognitive functioning in individual cognitive domains, such as executive functioning (White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017) or social cognition (Gur et al., Reference Gur, Moore, Calkins, Ruparel and Gur2017). In a recent review, Jonas et al. (Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024) provided a preliminary demonstration of cognitive performance deficits associated with symptom levels in externalizing, psychosis, fear (phobias), and anxious-misery domains in the PNC. However, no study to date has utilized the full neurocognitive battery from the PNC, applied a data-driven approach to model psychopathology, and examined transdiagnostic and distinct links between psychopathology and cognition. Here, we adopted a dimensional approach to assess variations in mental health symptoms using a bifactor model, with one general psychopathology factor and six domain-specific factors, and examined associations with domains of neurocognitive functioning using a well-validated test battery.
Methods
Participants
The sample was derived from the PNC (N = 9498), an NIMH-funded Grand Opportunity (GO) project (Calkins et al., Reference Calkins, Merikangas, Moore, Burstein, Behr, Satterthwaite and Gur2015, Reference Calkins, Moore, Merikangas, Burstein, Satterthwaite, Bilker and Gur2014; Gur et al., Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012; Satterthwaite et al., Reference Satterthwaite, Elliott, Ruparel, Loughead, Prabhakaran, Calkins and Gur2014). The present study is a secondary analysis of clinical and neurocognitive data from this cross-sectional study. Briefly, the PNC is a community-based sample of individuals aged 8–21 years enrolled in genomic studies at the Children's Hospital of Philadelphia (CHOP) who provided permission to be recontacted. Of 50 293 individuals recruited by the CHOP Center for Applied Genomics, 19 161 potentially eligible participants met inclusion criteria based on Electronic Medical Records (EMRs): (a) ages 8–21, (b) English proficiency, (c) good general health allowing completion of study procedures, (d) written informed consent/assent for re-contacting for future studies. Of potentially eligible participants, 13 598 were invited to participate, the remaining unreachable or unable to schedule. Of those invited, 9498 (64%) were enrolled and 9350 had complete data for analyses. Data collection lasted from 2009 to 2013. The sample here were 51.8% female (mean age 13.3 years [s.d. = 3.7]), 56.1% White, 32.7% Black, 11.3% Other racial category.
Following an overview of the study, written consent from participants age >18 years and assent and parental/legal guardian consent for individuals <18 were obtained. The study protocol was approved by CHOP and University of Pennsylvania institutional review boards.
Psychopathology assessment
Clinical assessments were described previously (Calkins et al., Reference Calkins, Merikangas, Moore, Burstein, Behr, Satterthwaite and Gur2015). Briefly, a computerized structured interview (GOASSESS), adapted from the NIMH Genetic Epidemiology Research Branch Kiddie-SADS for Affective Disorders and Schizophrenia (Merikangas, Avenevoli, Costello, Koretz, & Kessler, Reference Merikangas, Avenevoli, Costello, Koretz and Kessler2009), was administered to probands and collaterals (ages 8–17) or probands alone (ages 18–21). In addition to medical history and demographic information, the GOASSESS evaluates several psychopathology domains: mood (Major Depressive Episode, Manic Episode), anxiety (Generalized Anxiety, Separation Anxiety, Specific Phobia, Social Phobia, Panic, Agoraphobia, Obsessive-Compulsive, Post-traumatic Stress), Attention-Deficit/Hyperactivity, behavioral (Oppositional Defiant, Conduct), eating disorders (Anorexia, Bulimia), and suicidality. To evaluate negative/disorganized and positive psychosis symptoms, we used items from the Scale of Prodromal Symptoms (SOPS) from the Structured Interview for Prodromal Syndromes (McGlashan, Miller, & Woods, Reference McGlashan, Miller and Woods2003) and the Prevention through Risk Identification, Management, and Education (PRIME) Screen-Revised (Kobayashi et al., Reference Kobayashi, Nemoto, Koshikawa, Osono, Yamazawa, Murakami and Mizuno2008; Miller et al., Reference Miller, McGlashan, Rosen, Cadenhead, Ventura, McFarlane and Woods2003), respectively. Here, 115 screening items from the GOASSESS were used, but detailed probe items (e.g. duration of specific symptoms, functional impairment) were not considered because their inclusion resulted in unusual item properties due to very small variances. Assessments were conducted by Bachelor's and Master's level trained clinical research coordinators (n = 55) at the laboratory or the participant's home. Assessors underwent common training and certification protocols accompanied by periodic re-trainings, and all interview data underwent standardized post-administration review procedures (Calkins et al., Reference Calkins, Merikangas, Moore, Burstein, Behr, Satterthwaite and Gur2015). Each interview required the presence of one assessor. Participants' responses to interview questions were recorded in the database.
Cognitive assessment
Neurocognitive functioning was assessed using the Penn Computerized Neurocognitive Battery (CNB), which includes tests covering a broad array of cognitive domains (Gur et al., Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012, Reference Gur, Calkins, Satterthwaite, Ruparel, Bilker, Moore and Gur2014, Reference Gur, Richard, Hughett, Calkins, Macy, Bilker and Gur2010; Moore, Reise, Gur, Hakonarson, & Gur, Reference Moore, Reise, Gur, Hakonarson and Gur2015; Roalf et al., Reference Roalf, Gur, Ruparel, Calkins, Satterthwaite, Bilker and Gur2014). The CNB has been validated in populations with wide age ranges in clinical and community samples (Gur et al., Reference Gur, Kohler, Ragland, Siegel, Lesko, Bilker and Gur2006, Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012; Hartung et al., Reference Hartung, Kim, Laney, Hooper, Radcliffe, Port and Furth2016; Iannacone et al., Reference Iannacone, Leary, Esposito, Ruparel, Savitt, Mott and Abella2014; Irani et al., Reference Irani, Brensinger, Richard, Calkins, Moberg, Bilker and Gur2012; Roalf et al., Reference Roalf, Gur, Ruparel, Calkins, Satterthwaite, Bilker and Gur2014; Silver et al., Reference Silver, Goodman, Bilker, Gur, Isakov, Knoll and Feldman2006). All neurocognitive tests have been previously detailed (Gur et al., Reference Gur, Ragland, Moberg, Turner, Bilker, Kohler and Gur2001, Reference Gur, Richard, Hughett, Calkins, Macy, Bilker and Gur2010, Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012; Moore et al., Reference Moore, Reise, Gur, Hakonarson and Gur2015) and are summarized in the Supplement by cognitive domain. The 14 CNB tests take approximately 1-h to complete and assess five neurocognitive domains: complex cognition, executive function, episodic memory, social cognition, and sensorimotor speed. Clinical research coordinators trained on a standard protocol administered the CNB on the web-based platform (Gur et al., Reference Gur, Richard, Hughett, Calkins, Macy, Bilker and Gur2010, Reference Gur, Richard, Calkins, Chiavacci, Hansen, Bilker and Gur2012) and installed it on laptops for testing in participants' homes or the laboratory. Response times and responses were recorded and uploaded to the database.
Analyses
Demographic variables (age, sex) were obtained during the psychopathology assessment. Socioeconomic status (SES) was approximated by linking participants' addresses to census block-groups (neighborhoods) (Moore et al., Reference Moore, Martin, Gur, Jackson, Scott, Calkins and Gur2016).
Factor analyses were conducted to construct measurement models and examine the relations between latent psychopathology and cognition factors. To model psychopathology, we used the item-wise clinical data. For participants in the 8–10 age group, we used the collateral report, and for ages 11–21, we used proband report. While original analyses of these items (Shanmugan et al., Reference Shanmugan, Wolf, Calkins, Moore, Ruparel, Hopson and Satterthwaite2016) extracted four factors (and ‘p’), here we applied a data-driven approach to examine whether items might ‘split’ further into additional sub-factors (beyond 4), allowing relationships between psychopathology and cognition to be more specific. Indeed, we found two additional factors. This addition of factors was supported by the CFA fit indices: while the 4-factor model produced acceptable conventional fit indices (CFI, SRMR, and RMSEA), replicating Shanmugan et al. (Reference Shanmugan, Wolf, Calkins, Moore, Ruparel, Hopson and Satterthwaite2016), the ‘robust’ fit indices produced by Lavaan were unacceptable for the 4-factor model. It was not until six factors were specified that the ‘robust’ fit indices reached acceptable levels. In the present analysis, we split the sample and conducted a series of preliminary exploratory factor analyses (EFA) in the first half of the sample to determine the best underlying structure for the symptom domains. The 6-factor solution was then used in confirmatory factor analyses (CFA), which were conducted on the second half of the sample, with a bifactor configuration. Age, sex, and SES were controlled for in the measurement model at the item level. Factor reliability indices (e.g. H, determinacy, etc.) commonly reported for bifactor models (Rodriguez, Reise, & Haviland, Reference Rodriguez, Reise and Haviland2016) were estimated.
Cognition was modeled based on our previous work (Moore et al., Reference Moore, Reise, Gur, Hakonarson and Gur2015). Scores reflected efficiency, defined as the average of standardized speed and accuracy scores. Based on prior (2015) recommendation, our model placed abstraction and mental flexibility in the complex cognition factor instead of the executive control factor. The present model additionally differed from the Moore et al. (Reference Moore, Reise, Gur, Hakonarson and Gur2015) model by including two additional indicators: motor (tapping) speed and sensorimotor (praxis) speed. These scores were modeled as their own factor, resulting in a 5-factor model (compared to 4 factors in previous work). A second ad hoc correction made to the cognition model was that the bifactor configuration was abandoned in favor of a correlated-traits configuration (Reise et al., Reference Reise, Moore and Haviland2010), because the H indices of factor reliability (Rodriguez et al., Reference Rodriguez, Reise and Haviland2016) were too low for the specific factors in the bifactor model to justify relating them to the clinical factor in an SEM. The correlated-traits model fixed this problem, resulting in highly reliable latent cognitive factors in the CFA. Sex and SES were controlled for in all cognitive variables within the measurement model, as in the clinical model. Age was controlled for before analysis using age-normalization of scores. Sex, SES, and age were controlled for at the indicator-level (i.e. item- or test-level) within the confirmatory factor analysis; rather than relating the estimated factors to these covariates (regressing out of the factors), they were regressed out of the indicators of those factors.
Data imputation and EFAs were conducted in R v4.2.2 (R Core Team, 2022), using the missForest (Stekhoven, Reference Stekhoven2022; Stekhoven & Bühlmann, Reference Stekhoven and Bühlmann2012) and Lavaan (Rosseel, Reference Rosseel2012) packages, respectively. CFAs were conducted in Mplus version 8.4 (Muthén & Muthén, Reference Muthén and Muthén1998–2019) using the mean- and variance-adjusted weighted least squares (wlsmv) estimator (Muthén, du Toit, & Spisic, Reference Muthén, du Toit and Spisic1997). Model fit was evaluated based on recommendations from previous research (Hu & Bentler, Reference Hu and Bentler1998; Hu & Bentler, Reference Hu and Bentler1999). Bifactor-specific indices were calculated in R, using the BifactorIndicesCalculator package (Dueber, Reference Dueber2021). The effects of interest and the two models were estimated concurrently. Effects of interest were the relationships between clinical and cognitive factors. Factors within the clinical model were treated orthogonally, while cognitive factors were allowed to covary. All analyses covaried for age, sex, and SES.
Results
Exploratory factor analysis: clinical model
Supplementary Table 1 shows EFA results from one-half of the sample (n = 4674). The 6 factors represented the following domains: dysphoria/distress, obsessive-compulsive (OC), behavioral/externalizing, attention-deficit/hyperactivity (ADH), phobias, and psychosis. Next, CFA with a bifactor configuration to include an overall psychopathology (‘p’) factor was conducted in the second half of the sample, and model fit was acceptable. The comparative fit index (CFI) was 0.91 and the Tucker-Lewis index (TLI) was 0.90; the root mean-square error of approximation (RSMEA) was 0.025 and the standardized root mean square residual (SRMR) was 0.063. The strongest indicators of each factor are detailed in Supplement.
Compared to Shanmugan et al. (Reference Shanmugan, Wolf, Calkins, Moore, Ruparel, Hopson and Satterthwaite2016) 4-factor structure, our model revealed further separation in the previously defined ‘anxious-misery’ factor and the behavioral factor. Specifically, the anxious-misery (mood-anxiety symptoms) factor split, with OC diverging as its own factor. We labeled the remaining symptoms ‘dysphoria/distress’, aligning with HiTOP nomenclature (Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024; Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017). The ADH symptoms diverged from the previous behavioral factor into a distinct factor, and we labeled the remaining symptoms as ‘behavioral/externalizing’ factor, aligning with HiTOP nomenclature. The fear (phobias) and the psychosis factors remained.
Confirmatory factor analysis
To model cognition, we ran a correlated-traits model with the five domains: complex cognition, executive function, episodic memory, social cognition, and sensorimotor speed. The fit indices were adequate (CFI = 0.96; TLI = 0.92; RMSEA = 0.048; SRMR = 0.025).
The final confirmatory model, tested on the entire sample, included the cognitive correlated-traits model (Table S2) and the clinical bifactor model (Table S3), showing acceptable fit (CFI = 0.91; TLI = 0.90; RMSEA = 0.024; SRMR = 0.054). Significant associations among domains of the psychopathology and cognitive models are shown in Fig. 1. Cognitive factors were specified to correlate with clinical factors. Within the context of the final confirmatory model, the psychopathology bifactor model showed good internal consistency, supporting the presence of an overall psychopathology (‘p’) factor (general factor ω = 0.98; ωH = 0.88, H = 0.97), with sufficient reliability remaining in each specific factor (Hs = 0.80–0.86). While the bifactor is the least parsimonious measurement model (Reise et al., Reference Reise, Moore and Haviland2010), given the borderline fit of the bifactor model (e.g. CFI = 0.91), that added complexity appears to be necessary. See Supplementary Methods for greater detail and Supplementary Table S4 for bifactor reliability indices.

Figure 1. Structural equation model with psychopathology bifactor and cognitive correlated-traits models.
Note: The structural equation model depicts the significant associations (p < 0.05) between the latent psychopathology factors of the bifactor model and the latent cognitive factors in the correlated-traits model. Blue arrows indicate positive associations; red arrows indicate negative associations. OC, obsessive-compulsive; ADH, attention-deficit/hyperactivity.
Associations between clinical and cognitive domains
Cognition and psychopathology dimensions showed both factor-specific and overall significant associations (Table 1). The p-factor was associated with poorer complex cognition (p < 0.0005), but no other domain-specific deficit. All cognitive domains were negatively associated with ADH (all ps < 0.0005), phobias (all ps < 0.0005), and psychosis (ps<0.0005 for complex cognition, episodic memory, and social cognition; p = 0.006 for executive function; p = 0.048 for sensorimotor speed) factors, indicating that greater psychopathology symptom burden was associated with poorer cognitive performance. The behavioral/externalizing factor was negatively associated with complex cognition and executive functioning (ps < 0.0005), but with no other neurocognitive domain. Positive associations were observed between dysphoria/distress (p = 0.043) and OC (p = 0.005) factors and complex cognition, indicating that those with greater symptom burden had better complex reasoning abilities. However, these psychopathology dimensions did not show significant associations with other cognitive domains.
Table 1. Associations between neurocognitive and psychopathology domains

OC, obsessive-compulsive; ADH, attention-deficit/hyperactivity.
Boldface indicates significant effects (p < 0.05).
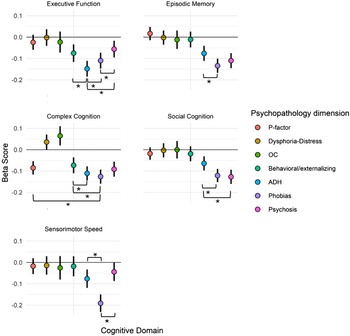
Differences in the magnitude of the associations between cognitive functioning and psychopathology dimensions also emerged. To assess the statistical significance of the differences among estimates, a 95% confidence interval (CI) for each estimate was calculated. Since some overlap in CIs does not necessarily indicate lack of significant differences between groups, we have indicated the significance of differences by asterisks in Fig. 2 and specified them in Supplementary Table S5. As can be seen in Fig. 2 and Table S5, psychopathology dimensions showed some specificity of association with cognitive performance. Overall, phobias, ADH, and psychosis were associated with deficits across domains. However, ADH showed the greatest deficit in executive functions, phobias had the greatest deficits in episodic memory, complex cognition, and sensorimotor speed, and psychosis had the greatest deficits in social cognition. The behavioral/externalizing factor was associated with the poorest performance specifically in executive functioning and complex cognition domains, and both OC and dysphoria/distress were associated with better than average complex cognition performance.

Figure 2. Effect sizes for psychopathology-cognitive domain associations profiles of psychopathology domain beta scores for each cognitive domain.
Note: Error bars represent 95% confident intervals. ADH, attention-deficit/hyperactivity; OC, obsessive-compulsive. * indicates significant difference between compared groups.
Discussion
Prior research has shown that neurocognitive deficits are transdiagnostic in nature (Abramovitch et al., Reference Abramovitch, Short and Schweiger2021). However, it has been challenging to precisely characterize the relationships between neurocognitive functioning and psychopathology dimensions to determine the specificity of such associations. Using bifactor and correlated-traits models, the present study evaluated relationships among domains of cognition and dimensions of psychopathology in a large, community-based youth cohort. The latent structure of the psychopathology model uncovered an overall psychopathology (‘p’) factor and 6 orthogonal symptom dimensions – dysphoria/distress, OC, behavioral/externalizing, ADH, phobias, and psychosis. We found both unique and shared patterns of associations between these psychopathology dimensions and five domains of neurocognitive functioning: executive function, episodic memory, complex cognition, social cognition, and sensorimotor speed. While our findings support the existence of transdiagnostic cognitive deficits, they also identify some specificity in associations with psychopathology dimensions, including dimensions associated with better neurocognitive performance.
Our results are consistent with extensive literature demonstrating a relationship between general psychopathology (‘p’) and cognition (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018; Brislin et al., Reference Brislin, Martz, Joshi, Duval, Gard, Clark and Sripada2022; Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014; Castellanos-Ryan et al., Reference Castellanos-Ryan, Brière, O'Leary-Barrett, Banaschewski, Bokde, Bromberg and Consortium2016; Kramer et al., Reference Kramer, Willcutt, Peterson, Pennington and McGrath2023; Martel et al., Reference Martel, Pan, Hoffmann, Gadelha, do Rosário, Mari and Salum2017; Romer & Pizzagalli, Reference Romer and Pizzagalli2021). However, our analyses revealed that only the complex cognition domain was associated with general psychopathology. Notably, most prior research linked executive functioning to the p-factor (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018; Castellanos-Ryan et al., Reference Castellanos-Ryan, Brière, O'Leary-Barrett, Banaschewski, Bokde, Bromberg and Consortium2016; Martel et al., Reference Martel, Pan, Hoffmann, Gadelha, do Rosário, Mari and Salum2017; Romer & Pizzagalli, Reference Romer and Pizzagalli2021; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017). Difference in results may reflect different definitions of cognitive constructs. Kramer et al. (Reference Kramer, Willcutt, Peterson, Pennington and McGrath2023) found that higher overall psychopathology was linked to poorer performance on processing speed tasks. However, certain tasks in their battery measure aspects of complex cognition. For example, the Colorado Speed Test (Decker, Reference Decker1989) and the Penn Verbal Reasoning Test (PVRT) relate to abstraction and language. Similarly, White et al. (Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017) assessed executive functioning using the Penn Conditional Exclusion Test (PCET), included in our complex cognition domain to align with prior findings (Moore et al., Reference Moore, Reise, Gur, Hakonarson and Gur2015). The effect sizes we found are consistent with prior studies that also found small correlations. The extensive original study (Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014) found relatively weak relationships between ‘p’ and cognition/IQ (e.g. −0.19 for WAIS FSIQ, −0.13 for verbal comprehension, −0.13 for perceptual reasoning, −0.18 for working memory, etc.), and other studies (e.g. Castellanos-Ryan et al., Reference Castellanos-Ryan, Brière, O'Leary-Barrett, Banaschewski, Bokde, Bromberg and Consortium2016) had similarly small effects (r = 0.07–0.14). The small effect sizes in the present study are perhaps due to use of efficiency rather than accuracy as the main cognitive dimension, and correction for several covariates (SES, gender, age) at the test and item level (compared to uncorrected bivariate correlations in Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014).
Though prior reviews have suggested that psychopathology is associated with generalized cognitive deficits (Abramovitch et al., Reference Abramovitch, Short and Schweiger2021), our findings suggest some specificity beyond transdiagnostic cognitive dysfunction. Consistent with prior work, higher levels of phobias, psychosis, behavioral/externalizing, and ADH symptoms were associated with lower performance in executive functioning and complex cognition (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018; Gur et al., Reference Gur, Calkins, Satterthwaite, Ruparel, Bilker, Moore and Gur2014; Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024; Kramer et al., Reference Kramer, Willcutt, Peterson, Pennington and McGrath2023; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017). Higher levels of phobias, psychosis, and behavioral symptoms were also associated with lower performance in episodic memory. Lower performance in social cognition tasks was likewise reported for psychosis (Gur et al., Reference Gur, Kohler, Ragland, Siegel, Lesko, Bilker and Gur2006, Reference Gur, Moore, Calkins, Ruparel and Gur2017; Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024), phobias (Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024; Plana, Lavoie, Battaglia, & Achim, Reference Plana, Lavoie, Battaglia and Achim2014), and ADH dimensions (Parke et al., Reference Parke, Becker, Graves, Baily, Paul, Freeman and Allen2021; Uekermann et al., Reference Uekermann, Kraemer, Abdel-Hamid, Schimmelmann, Hebebrand, Daum and Kis2010). Regarding sensorimotor speed, we replicated an association between slower sensorimotor speed and psychosis symptoms (Osborne, Walther, Shankman, & Mittal, Reference Osborne, Walther, Shankman and Mittal2020). We also found that the phobias and ADH factors were associated with slower motor speed, but the nature of this relationship is less clear (Farran et al., Reference Farran, Bowler, D'Souza, Mayall, Karmiloff-Smith, Sumner and Hill2020). Finally, OC and dysphoria/distress dimensions were associated with better performance in complex cognition (Brislin et al., Reference Brislin, Martz, Joshi, Duval, Gard, Clark and Sripada2022; Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024). Such specificities may have been masked in prior studies due to smaller samples, limited psychopathology dimensions, or a reduced neurocognitive battery. Our model's parsing of psychopathology factors and examination of a broad range of cognitive functions may offer more sensitivity to domain-specific associations with cognition.
Psychosis was linked more strongly to poorer social cognition than ADH, consistent with prior PNC research showing that psychosis is associated with lower social cognition scores compared to other dimensions (Gur et al., Reference Gur, Moore, Calkins, Ruparel and Gur2017). On the other hand, phobias and ADH were associated with even poorer executive functioning than psychosis, diagnostic domains that need comparative examination (Service et al., Reference Service, Vargas Upegui, Castaño Ramírez, Port, Moore, Munoz Umanes and Freimer2020; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017). Consistent with prior research (Clark, Prior, & Kinsella, Reference Clark, Prior and Kinsella2000; Ezpeleta & Granero, Reference Ezpeleta and Granero2015; Jarrett, Reference Jarrett2016), ADH was more strongly linked to executive function than the phobias or behavioral/externalizing dimensions. The phobias factor was significantly associated with all neurocognitive domains. Furthermore, it was more strongly linked to poorer complex cognition than the behavioral dimension and overall psychopathology, and more strongly associated with poorer episodic memory and social cognition than ADH. We also found that phobias had a more robust association with sensorimotor speed compared to ADH and psychosis. Among all cognitive domains, sensorimotor speed was most strongly linked to phobias, a finding that could be pursued in future studies. Prior work has reported mixed associations between phobias and cognitive functioning (Demetriou et al., Reference Demetriou, Park, Pepper, Naismith, Song, Thomas and Guastella2021; Gustavson & Miyake, Reference Gustavson and Miyake2016; White et al., Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017). Further work may be needed to disentangle the covariation between phobias/fear and dimensions of personality or psychopathology, such as neuroticism or negative emotionality (Watson, Clark, Simms, & Kotov, Reference Watson, Clark, Simms and Kotov2022).
The dimensions significantly associated with poorer neurocognitive functioning across all domains were psychosis and ADH – the latter collapsed in the behavioral factor in prior studies (Calkins et al., Reference Calkins, Merikangas, Moore, Burstein, Behr, Satterthwaite and Gur2015; Shanmugan et al., Reference Shanmugan, Wolf, Calkins, Moore, Ruparel, Hopson and Satterthwaite2016). Both psychosis and behavioral/ADH are often extracted in dimensional studies of psychopathology (Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel and Moffitt2014; Martel et al., Reference Martel, Pan, Hoffmann, Gadelha, do Rosário, Mari and Salum2017; Parkes et al., Reference Parkes, Moore, Calkins, Cook, Cieslak, Roalf and Bassett2021; Sunderland et al., Reference Sunderland, Forbes, Mewton, Baillie, Carragher, Lynch and Slade2021). Additionally, they can be related to components of the p-factor identified by Southward, Cheavens, and Coccaro (Reference Southward, Cheavens and Coccaro2023). Specifically, ADH has been linked to impulsivity (Leffa, Caye, & Rohde, Reference Leffa, Caye, Rohde, Stanford and Sciberras2022; Walerius, Reyes, Rosen, & Factor, Reference Walerius, Reyes, Rosen and Factor2018), while psychosis-related disorders can be conceptualized as thought disorders (Kotov et al., Reference Kotov, Krueger, Watson, Achenbach, Althoff, Bagby and Zimmerman2017). Impulsivity has been proposed as a main subcomponent of the p-factor, and thought dysfunction has shown a strong link with the p-factor (Southward et al., Reference Southward, Cheavens and Coccaro2023). Examining how dimensions intersect with cognitive performance across various domains could allow a more precise characterization of at-risk individuals and uncover potential avenues for targeted interventions.
Dysphoria/distress and OC factors were associated with better performance on complex cognition tasks, similar to prior work and a recent demonstration of cognitive functioning associated with anxious-misery symptoms in the PNC (Jonas et al., Reference Jonas, Cannon, Docherty, Dwyer, Gur, Gur and Kotov2024). Increased internalizing symptoms, typically including anxious/depressed and somatic complains, have been linked to higher general cognitive functioning (Brislin et al., Reference Brislin, Martz, Joshi, Duval, Gard, Clark and Sripada2022) and cognitive flexibility (Bloemen et al., Reference Bloemen, Oldehinkel, Laceulle, Ormel, Rommelse and Hartman2018). Of note, White et al. (Reference White, Moore, Calkins, Wolf, Satterthwaite, Leibenluft and Gur2017), who included the PCET in the executive function domain in the PNC, also reported a positive association between executive functioning and the anxious-misery symptom dimension. Our findings also replicate Service et al.'s (Reference Service, Vargas Upegui, Castaño Ramírez, Port, Moore, Munoz Umanes and Freimer2020) transdiagnostic study of the Paisa population in Colombia. The major depression group was the least impaired across neurocognitive domains, and the ‘anxious-misery’ factor, across diagnoses, was positively associated with performance, especially on social cognition.
Limitations of the present study should be acknowledged. The sample was restricted to individuals aged 8–21, affecting generalizability to other age groups. Relatedly, clinical data for the psychopathology models were drawn from collateral information for participants aged 8–10 years and proband information for those aged 11–21 years, similar to prior work. However, this discrepancy could affect the validity and reliability of results, given that divergence between proband and collateral reports have been reported across psychopathology domains, race, and SES (e.g. Belendiuk, Clarke, Chronis, and Raggi, Reference Belendiuk, Clarke, Chronis and Raggi2007; Curhan, Rabinowitz, Pas, and Bradshaw, Reference Curhan, Rabinowitz, Pas and Bradshaw2020; Jones et al., Reference Jones, Scott, Calkins, Ruparel, Moore, Gur and Gur2017; Salbach-Andrae, Klinkowski, Lenz, and Lehmkuhl, Reference Salbach-Andrae, Klinkowski, Lenz and Lehmkuhl2009; Xavier et al., Reference Xavier, Calkins, Bassett, Moore, George, Taylor and Gur2022). In addition, the PNC evaluated lifetime diagnoses in a community-based, non-clinical sample, which might assess psychopathology traits rather than states. This is perhaps why higher p-factor scores significantly associated only with poorer complex cognition performance, the most stable, trait-like factor in the CNB (analogous to IQ; Beaver et al., Reference Beaver, Schwartz, Connolly, Nedelec, Al-Ghamdi and Kobeisy2013). The focus on lifetime diagnoses rather than current symptoms also might not have fully captured the temporal aspect of certain conditions. Certain domains of psychopathology, such as personality disorders and substance use disorders, were not assessed. The use of cross-sectional data hinders establishing the directionality of the relationships between cognitive domains and symptom dimensions (Abramovitch et al., Reference Abramovitch, Short and Schweiger2021; Bredemeier et al., Reference Bredemeier, Warren, Berenbaum, Miller and Heller2016; Letkiewicz et al., Reference Letkiewicz, Miller, Crocker, Warren, Infantolino, Mimnaugh and Heller2014; Romer & Pizzagalli, Reference Romer and Pizzagalli2021). The sub-factors in the cognition model might be ‘contaminated’ with the ‘g’ factor since we did not use a bifactor model. We used efficiency scores in these analyses rather than examining performance accuracy and speed separately, which prevents specification of specific associations of psychopathology with accuracy or speed individually. Given that our sample spanned ages marked by significant neurodevelopment, it is possible that the associations between cognitive and clinical factors are not consistent across the age range, or that factors used are not invariant across this age range; future work should examine this possibility. Finally, to model psychopathology we used a bifactor model, which has been criticized for overfitting data and undermining model validity (Watts, Lane, Bonifay, Steinley, & Meyer, Reference Watts, Lane, Bonifay, Steinley and Meyer2020).
Notwithstanding these limitations, the present study offers insights into the transdiagnostic nature of cognitive deficits in youth. Findings support both transdiagnostic cognitive deficits and variability in the link between clinical phenotypes and neurocognitive functioning. Future research should explore these relationships in a sample with a broader age range and more severe clinical profiles, including elevated current symptoms. Longitudinal studies exploring the temporal link between psychopathology and cognitive deficits could also inform treatment and early interventions targeting cognition to prevent, detect, or treat mental health conditions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724001302.
Acknowledgements
We thank study participants and their families as well as Dr Hakon Hakonarson and the research team for their efforts and contributions to this study.
Funding statement
This work was supported by the National Institute of Mental Health (R.C.G. and R.E.G., grant numbers MH119219, MH117014), (R.E.G. and H.H., grant numbers MH089983, MH089924), (M.E.C., grant number K08MH079364), VA Office of Research and Development (J.C.S., grant number I01RX002699), and the Penn-CHOP Lifespan Brain Institute. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
Competing interests
The authors declare none.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.