Introduction
Of all cognitive domains, verbal memory is one of the most frequently and severely affected in schizophrenia (Heinrichs & Zachzanis, Reference Heinrichs and Zakzanis1998; Aleman et al. Reference Aleman, Hijman, de Haan and Kahn1999); the deficit is present at all stages of the illness (Saykin et al. Reference Saykin, Shtasel, Gur, Kester, Mozley, Stafiniak and Gur1994) and has been implicated as a mediator of poor clinical outcome (Green et al. Reference Green, Kern, Braff and Mintz2000). However, the exact nature of the verbal memory deficit is still not established. Whereas there is little doubt that schizophrenia patients demonstrate encoding deficits, manifest as poor learning, it is still unclear whether retention of verbal material is impaired (see Cirillo & Seidman, Reference Cirillo and Seidman2003). When delayed recall is corrected for initial learning, some studies (Toulopoulou et al. Reference Toulopoulou, Rabe-Hesketh, King, Murray and Morris2003; Nuyen et al. Reference Nuyen, Sitskoorn, Cahn and Kahn2005; Chan et al. Reference Chan, Chen and Law2006; Rametti et al. Reference Rametti, Segarra, Junque, Bargallo, Caldu, Ibarretxe and Bernardo2007) but not others (Holthausen et al. Reference Holthausen, Wiersma, Sitskoorn, Dingemans, Schene and Van den Bosch2003; Kristian Hill et al. Reference Kristian Hill, Beers, Kmiec, Keshavan and Sweeney2004; Lee et al. Reference Lee, Chan, Chan, Gao, Wang and Chen2006; Roofeh et al. Reference Roofeh, Cottone, Burdick, Lencz, Gyato, Cervellione, Napolitano, Kester, Anderson and Kumra2006) find an effect of delay on free recall. In those studies finding impaired delayed recall, it is unclear whether this represents a failure of retrieval of stored information or a failure of storage per se.
It is important to distinguish between the component processes contributing to memory impairment in schizophrenia because they are subserved by different neural processes and this has implications for understanding the neurobiology of the disorder. For example, impaired encoding and retrieval implicates involvement of prefrontal cortex (Fletcher & Henson, Reference Fletcher and Henson2001) whereas accelerated forgetting implies impaired consolidation attributable to medial temporal lobe (MTL) dysfunction (Alvarez & Squire, Reference Alvarez and Squire1994).
Another reason for dissecting memory performance and its relationship to other forms of cognitive dysfunction is because of the controversy concerning the nature of cognitive impairment in schizophrenia. As most neuropsychological studies find wide-ranging impairments (e.g. Mohamed et al. Reference Mohamed, Paulsen, O'Leary, Arndt and Andreasen1999; Bilder et al. Reference Bilder, Goldman, Robinson, Reiter, Bell, Bates, Pappadolulos, Wallson, Alvir, Woerner, Geisler, Kane and Lieberman2000), an important question is whether this represents multiple independent and possibly differential impairments of specific cognitive processes (Nuechterlein et al. Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004) or whether schizophrenia is best characterized by a generalized cognitive impairment varying from person to person in degree (Dickinson et al. Reference Dickinson, Iannone, Wilk and Gold2004). In particular, under many circumstances, episodic encoding and retrieval entail cognitive control processes that affect the ability to plan, initiate strategies and inhibit distractions (Ranganath et al. Reference Ranganath, Minzenberg and Ragland2008), and therefore it is important to determine whether memory deficits can occur independently of executive dysfunction in schizophrenia.
We have addressed these questions in large groups of healthy volunteers and first-episode psychosis patients. First, we investigated the various subcomponents of episodic memory. Second, we compared performance of verbal memory measures with measures of visuospatial executive function and general ability. Our hypothesis was that measures of episodic memory would be specifically and strongly associated with executive function performance, indicating the primacy of prefrontal cortex impairment in the neurobiology of schizophrenia.
Method
Subjects
Patients were recruited as part of a study of first-episode psychosis in West London. Those eligible presented from the community to mental health services with a psychotic illness for the first time and had no more than 12 weeks cumulative exposure to antipsychotic medication. Data from 97 patients were included on the basis that they had received an initial diagnosis of schizophrenia, schizophreniform or schizo-affective disorder, completed all neuropsychological assessments and undertaken clinical assessments sufficient to make a final diagnosis. A follow-up clinical assessment was performed on all but 14 patients at least 1 year following first presentation. Of those patients who declined or were unavailable for reassessment, follow-up clinical information including diagnoses was obtained from clinical case-notes for 12 patients. The diagnoses for the remaining two patients were based on current clinical state and duration of illness. The final DSM-IV diagnoses were schizophrenia in 86 and schizo-affective disorder in 11. At the time of assessment, five were medication free, 15 were receiving first-generation antipsychotics, 75 second-generation antipsychotics and two a combination of both; 11 were taking anticholinergics.
Ninety-seven healthy volunteers served as controls, recruited by advertising in local job centres, schools and hospitals. Exclusion criteria were a personal history of psychiatric illness or a history of such illness in any first-degree relatives, previous head injury, neurological or endocrine disorder known to affect brain function, and drug or alcohol abuse. Table 1 contains demographic information on both groups.
Table 1. Demographic and cognitive profiles of the patient and control groups
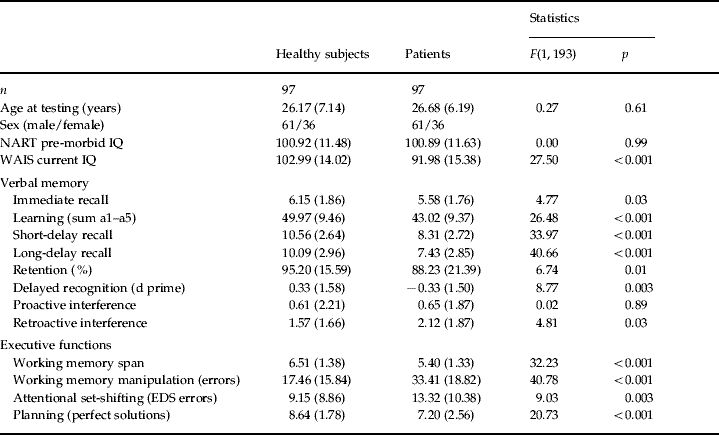
Values given as mean (standard deviation).
NART, National Adult Reading Test; WAIS, Wechsler Adult Intelligence Scale; EDS, extra-dimensional shift.
The patient and control groups were taken from larger groups of 173 psychotic patients and 144 controls on the basis that they could be one-to-one matched on National Adult Reading Test (NART) pre-morbid IQ (68 were exactly the same, 25 were within one IQ point and four were within two IQ points) and sex. Age was also matched as closely as possible.
Permission to conduct the study was obtained from the relevant Research Ethics Committees. All participants gave written informed consent and were paid an honorarium for their time. A subset of the cognitive data from 31 patients and 17 controls has been reported previously (Joyce et al. Reference Joyce, Hutton, Mutsatsa and Barnes2005).
Clinical assessments
Psychotic symptoms were assessed with the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS; Andreasen, Reference Andreasen1983, Reference Andreasen1984). Scores for the three symptom-derived syndromes of schizophrenia (Liddle & Barnes, Reference Liddle and Barnes1990) were calculated for each patient. Depression was assessed in 60 patients with the Hamilton Depression Rating Scale (HAMD; Hamilton, Reference Hamilton1960). The dates of onset of psychosis were elicited as reported previously (Barnes et al. Reference Barnes, Hutton, Chapman, Mutsatsa, Puri and Joyce2000) to calculate the duration of untreated psychosis (DUP). Pre-morbid function was assessed using the scales for Premorbid Social Adjustment (PSA) and Premorbid Schizotypal Traits (PSST) (Foerster et al. Reference Foerster, Lewis, Owen and Murray1991).
Neuropsychological assessments
Cognitive assessments were performed when the patients were clinically stable as judged by the clinical team; this was within 1 week of the initial clinical assessment for 38% and 4 weeks for 78%. The remainder were tested within 2 months except four patients tested at 11, 11, 23 and 24 weeks. Pre-morbid IQ was estimated using the Revised NART (Nelson & Willison, Reference Nelson and Willison1991). Current IQ was calculated from four subtests of the Wechsler Adult Intelligence Scale – Revised (WAIS-R: 37 patients, 17 controls; Wechsler, Reference Wechsler1981) or the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III; 60 patients, 80 controls; Wechsler, Reference Wechsler1997), which have been shown to provide reliable measures of full-scale IQ in psychosis (Missar et al. Reference Missar, Gold and Goldberg1994; Blyler et al. Reference Blyler, Gold, Iannone and Buchanan2000). These subtests were information, arithmetic, block design and digit symbol from the WAIS-III and information, similarities, picture completion and digit symbol from the WAIS-R. The patients tested using the WAIS-R did not differ from those tested with the WAIS-III [F(1, 95)=0.98, p=0.325].
Executive function was measured using the Cambridge Automated Neuropsychological Test Battery (CANTAB; Sahakian & Owen, Reference Sahakian and Owen1992). Working memory spatial span (Owen et al. Reference Owen, Downes, Sahakian, Polkey and Robbins1990) was measured by the ability to remember the order of sequences of squares presented on the screen in increasing number. Spatial working memory manipulation (Owen et al. Reference Owen, Downes, Sahakian, Polkey and Robbins1990) was measured by the number of errors made on a task in which subjects ‘opened’ sets of boxes, varying between three and eight in number, to find tokens. Planning (Owen et al. Reference Owen, Downes, Sahakian, Polkey and Robbins1990) was measured on a task where subjects moved coloured ‘balls’ in an arrangement on the screen to match a goal arrangement in problems differing in difficulty; accuracy was measured as the number of perfect solutions. Attentional set-shifting (Owen et al. Reference Owen, Roberts, Polkey, Sahakian and Robbins1991) was measured in a task where subjects learned a series of visual discriminations in which one of two stimulus dimensions was relevant. On the penultimate extra-dimensional shift (EDS) stage, the rule was reversed so that a previously irrelevant dimension now became relevant. Number of errors at this stage assessed the ability to inhibit the previously correct response set by shifting attention from one dimension to another.
Verbal memory was measured with the Rey Auditory Verbal Learning Task (RAVLT; Lezak, Reference Lezak1995). In trials 1–5, subjects were read the same list of 15 nouns and asked to recall as many as possible immediately afterwards. In trial 6, a second list was read and recalled. In trial 7 (short-delay), the original list was recalled without the list being read. After 25–30 min had elapsed, filled with performance of the executive tasks, the participants were asked to recall the original list again without the list being read (long-delay, trial 8). Trial 9 was a recognition memory trial. Extracted variables were: immediate memory (trial 1 recall), total number of words recalled during learning over trials 1–5 (learning), short-delay free recall (trial 7), long-delay free recall (trial 8) and recognition (trial 9 hit rate and false alarms rate). d prime was calculated using the hit and false alarm rates from the recognition trial [z(hit rate) – z(false alarm rate)] to provide an index of the subjects' ability to detect correct words while accounting for any bias in responding. We also calculated a savings score for memory retention as the percentage of items recalled on long-delay trial 8 as a function of the score on short-delay trial 7. A savings measure allows retention to be assessed more independently of performance over the learning trials than a simple delayed recall trial score (Seidman et al. Reference Seidman, Stone, Jones, Harrison and Mirsky1998). Two interference measures were calculated: proactive interference (the degree to which old material impairs the acquisition of new material, measured as trial 6 less trial 1) and retroactive interference (the degree to which new material impedes the retrieval of previously learned material, measured as trial 5 less trial 7).
Analyses
Data were analysed using SPSS version 15 (SPSS Inc., Chicago, IL, USA) and Mplus 4 (Muthén & Muthén, USA). ANOVA or ANCOVA was used for group comparisons. Categorical data were analysed using χ2. Separately for each group, the scores were z transformed to standardize scaling. Pearson's r correlations were performed to determine relationships between measures. An exploratory factor analysis (EFA) using unweighted least squares was performed to determine the best factor structure, with orthogonal rotation and loadings over 0.25 being retained. Both factor structures were then tested in the group from which they had been produced and the other group using confirmatory factor analyses (CFA).
Results
Table 1 shows that the matching procedure resulted in there being no significant differences in age, sex or pre-morbid IQ between the groups. Current WAIS IQ was significantly different between the groups; all executive and memory measures, with the exception of proactive interference, were also different. Z-score transformations of raw data, calculated in relation to the mean and standard deviation of the control, were performed for each trial of the auditory verbal learning test and are shown in Fig. 1. This demonstrates the extent to which the patients underperformed on verbal learning and memory compared to the matched controls.

Fig. 1. Mean patient performance on each stage of the auditory verbal learning task, shown as z scores transformed to the control data.
To examine the effect of learning capacity on free recall, we compared the two groups on short-delay free recall (trial 7) while covarying for learning (sum trials 1–5) using ANCOVA. This showed that, despite learning being a highly significant covariate [F(1, 191)=191.13, p<0.001], the difference between the groups remained significant once this was accounted for [F(1, 191)=8.38, p=0.004]. We repeated this analysis on long-delay free recall (trial 8) and, again, learning was a highly significant covariate [F(1, 191)=164.53, p<0.001] and the difference between the groups remained significant [F(1, 191)=13.466, p<0.001]. Examining long-delay free recall with both learning and short-delay free recall as covariates showed learning [F(1, 190)=12.29, p=0.001] and short-delay recall [F(1, 190)=143,85, p<0.001] to be significant covariates and that the difference between the groups for long-delay free recall remained significant [F(1, 190)=5.24, p=0.023]. When we examined the effect of learning on recognition memory, learning was a significant covariate as before [F(1, 191)=98.75, p<0.001] and the difference between groups in recognition score was no longer significant with this accounted for [F(1, 191)=1.57, p=0.212].
To examine the relationship between verbal learning and memory and other cognitive functions, correlations between measures were determined for each group (see Table 2). Factor analyses were performed using the z-score transformations of current IQ, verbal learning, verbal memory retention, working memory span, working memory manipulation, planning and attentional set-shifting.
Table 2. Pearson's r correlation matrix of cognitive measures in (a) controls and (b) patients

WAIS, Wechsler Adult Intelligence Scale.
a Error scores have been inverted.
* Significant at p<0.05, ** significant at p<0.01, *** significant at p<0.001.
Factor analyses
EFA revealed that a three-factor model was most appropriate in the control group (see Table 3 for factor loading pattern and eigenvalues). CFA of this model in the control group showed that it was a good fit [χ2=10.38, df=8, p=0.239, Comparative Fit Index (CFI)=0.978, Bayesian Information Criterion (BIC)=1924.98, root mean square error of approximation (RMSEA)=0.055, 90% confidence interval (CI) 0.00–0.139] although current IQ loaded negatively on the first factor. Therefore, other models were tested, including simple structure models (where measures that loaded onto more than one factor were only assigned to the factor onto which they loaded the most) and models with current IQ allowed to load onto the first or second factor. The best model allowed current IQ to load onto the second factor only and this variation was retained (χ2=10.58, df=9, p=0.306, CFI=0.985, BIC=1920.60, RMSEA=0.043, 90% CI 0.00–0.127. The factors correlated as follows: f1 and f2 0.45, f1 and f3 0.06, f2 and f3 0.11). When the same three-factor model was tested on the patient group it was a poor fit (χ2=18.35, df=9, p=0.031, CFI=0.94, BIC=1879.48, RMSEA=0.104, 90% CI 0.030–0.171. The factors correlated as follows: f1 and f2 0.49, f1 and f3 0.03, f2 and f3 0.7). Unweighted least squares factor analysis of the patient group revealed that a one-factor model was the most appropriate although verbal memory retention did not load onto this factor (see Table 3 for factor loading pattern and eigenvalues). CFA of this model with verbal memory retention omitted in the patient group showed a good fit (χ2=13.40, df=9, p=0.145, CFI=0.970, BIC=1578.43, RMSEA=0.071, 90% CI 0.00–0.145). In the control group the model was a reasonably good fit but less so than the three-factor model (χ2=15.75, df=9, p=0.072, CFI=0.928, BIC=1634.49, RMSEA=0.088, 90% CI 0.00–0.158).
Table 3. Orthogonal (Varimax) rotated factor loading patterns for the patient and control groups using exploratory factor analysis (unweighted least squares)
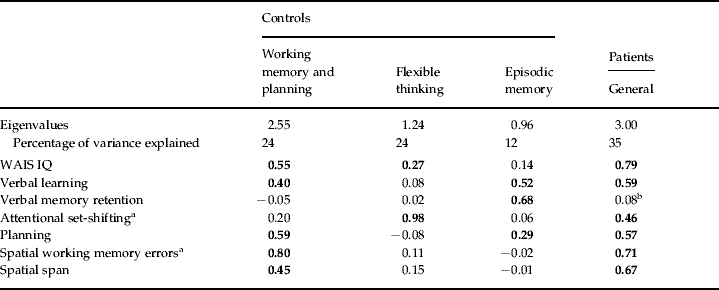
WAIS, Wechsler Adult Intelligence Scale.
Retained loadings over 0.25 are in bold face.
a Error scores have been inverted.
b Removed for confirmatory factor analysis (CFA).
Given the results of the previous analyses, to examine the degree to which verbal memory retention could predict group membership, we used data from both groups in a logistic regression with group membership (patient, control) as the binary dependent variable. Current IQ, verbal learning, working memory span, working memory manipulation, planning and attentional set-shifting were entered in a first block as predictors. This block was highly significant (χ2=51.50, p<0.001, df=6). Verbal memory retention was added in a second block and predicted a significant amount of the remaining variance (χ2=3.90, p=0.048, df=1).
Finally, we examined the relationship between verbal memory and clinical factors that might explain impaired verbal memory in the patient group (Paulsen et al. Reference Paulsen, Heaton, Sadek, Perry, Delis, Braff, Kuck, Zisook and Jeste1995): syndrome scores, depression, DUP, age at onset, and pre-morbid function where available (PSA and PSST). No correlations were significant with Bonferroni correction (range of r's −0.02 to 0.16). There were no differences between groups taking first- or second-generation medication (range of t's 0.01–1.41) or between groups taking anticholinergic medication or not (range of t's 0.12–0.62).
Discussion
In this study, patients with schizophrenia were impaired, relative to pre-morbid IQ-, sex- and age-matched healthy controls, at all stages of a verbal list learning task assessing immediate recall, learning, short- and long-delay free recall, and recognition. Studies of schizophrenia have frequently reported verbal memory deficits using list learning tasks and the deficits observed in this study are commensurate with the majority of these (see Cirillo & Seidman, Reference Cirillo and Seidman2003). Deconstructing the relative contribution of encoding, retrieval and retention to the verbal memory deficit is difficult given their interdependence. Patients show defective strategic processes at encoding, for example by failing to spontaneously organize the to-be-remembered material (e.g. Bonner-Jackson et al. Reference Bonner-Jackson, Yodkovik, Csernansky and Barch2008) and their recall improves when given organizational strategies (Ragland et al. Reference Ragland, Gur, Valdez, Loughead, Elliott, Kohler, Kanes, Siegel, Moelter and Gur2005). Furthermore, schizophrenia patients do not demonstrate greater facilitation when retrieval cues are presented (Brebion et al. Reference Brebion, Amador, Smith and Gorman1997). These findings have been taken as evidence that underperformance during learning is due to encoding deficits (Cirillo & Seidman, Reference Cirillo and Seidman2003) and our finding of poor recall on the first trial and impaired learning over subsequent trials supports this. Failure to adopt strategic encoding when learning word lists would also explain our finding of significantly greater retroactive interference effect on free recall of the initial list following presentation of a distractor word list (Craik, Reference Craik2002; Blumenfeld & Ranganath, Reference Blumenfeld and Ranganath2007).
However, encoding difficulties do not entirely explain the poor performance of the patients later in the task. When we controlled for learning, patients were still significantly worse than controls on the short-delay recall trial; when we controlled for both learning and short-delay recall, there remained a significant difference between the groups on long-delay recall. Furthermore, the memory retention measure, which assessed savings during the 30-min interval between short- and long-delay recall trials revealed that patients retained fewer words over the long delay. This supports the notion of faster forgetting in this group and is in keeping with several other studies (Toulopoulou et al. Reference Toulopoulou, Rabe-Hesketh, King, Murray and Morris2003; Nuyen et al. Reference Nuyen, Sitskoorn, Cahn and Kahn2005; Chan et al. Reference Chan, Chen and Law2006; Rametti et al. Reference Rametti, Segarra, Junque, Bargallo, Caldu, Ibarretxe and Bernardo2007). Thus, our findings support the conclusion of a comprehensive review (Cirillo & Seidman, Reference Cirillo and Seidman2003), that there is ‘mild but significantly impaired’ retention in schizophrenia. Overall, the results suggest that there are both encoding and retention difficulties in patients with schizophrenia at illness onset.
Our finding that long-delay recognition but not recall memory was intact in patients once the influence of learning was accounted for might seem problematic for this conclusion as it suggests that the learned verbal material was available for recall, thus implicating a retrieval deficit. The majority of previous studies comparing free recall and recognition in schizophrenia also find intact or relatively preserved verbal recognition memory (Aleman et al. Reference Aleman, Hijman, de Haan and Kahn1999; Cirillo & Seidman, Reference Cirillo and Seidman2003). One issue here is that the RAVLT, similar to other list learning tests, requires respondents to recall the same words presented for recognition. Recognition performance is likely to be influenced by prior recall and this may have explained why recognition was no longer significantly different between groups once recall trial performance was accounted for. Nevertheless, the same influence of previous recall trials would be expected to impact on delayed recall and this remained different between groups.
Recognition is not as dependent on the integrity of the memory trace as free recall. Recent evidence suggests that recognition reflects two independent processes: recollection (as in free recall) and familiarity (Aggleton & Brown, Reference Aggleton and Brown2006). There is growing evidence that familiarity judgements are intact in schizophrenia and that, in the presence of impaired recollection, recognition memory is reliant on familiarity judgements (van Erp et al. Reference van Erp, Lesh, Knowlton, Bearden, Hardt, Karlsgodt, Shirinyan, Rao, Green, Subotnik, Nuechterlein and Cannon2008a, Reference van Erp, Therman, Pirkola, Tuulio-Henriksson, Glahn, Bachman, Huttunen, Lönnqvist, Hietanen, Kaprio, Koskenvuo and Cannonb). Thus, impaired free recall and intact recognition does not necessarily imply an explanation solely in terms of deficient retrieval processes.
Kirwan et al. (Reference Kirwan, Wixted and Squire2008) report that activity in the MTL predicts memory strength whereas prefrontal cortex activity predicts recollection. Recollection may be impaired in contrast to familiarity because it is reliant on the prefrontal cortex, presenting as impaired recall but not recognition. However, correlation matrices of the neuropsychological measures used in this study revealed that verbal memory retention was not significantly correlated with several executive measures, suggesting that it is relatively independent.
To further understand the relationships between memory, executive function and general ability, we performed a factor analysis on current IQ, verbal learning and memory and measures of working memory span and manipulation, planning and attentional set-shifting. In the controls, three factors were produced corresponding to spatial working memory/planning, set-shifting and episodic memory. Separation by factor analysis of CANTAB measures of attentional set-shifting on the one hand and spatial span, spatial working memory and planning on the other has previously been found in a study of healthy ageing, which also showed that these two factors were distinct from a third factor containing measures of episodic memory (Robbins et al. Reference Robbins, James, Owen, Sahakian, Lawrence, McInnes and Rabbitt1998). Thus, the factor structure we obtained for controls seems consistent. The observation that IQ loaded primarily on the factors of working memory and flexible thinking is in keeping with the high degree of correlation between general ability and these executive functions in normal populations (see Blair, Reference Blair2006).
The patients showed a different pattern on factor analysis of the same variables in that all variables, except verbal memory retention, loaded onto a single factor. Verbal memory retention was completely uncorrelated with the other measures in this group. This suggests that, in patients with schizophrenia, verbal learning but not memory retention is highly related to executive function and supports the view that prefrontal cortex dysfunction is significantly associated with episodic memory encoding and probably underpins the learning deficits in schizophrenia (Fletcher & Henson, Reference Fletcher and Henson2001). Verbal memory retention, being independent of this relationship, may be a better index of MTL function (Alvarez & Squire, Reference Alvarez and Squire1994).
A second interpretation of the factor analysis findings is that cognitive function in schizophrenia is more generalized than normal. Whereas there were three factors explaining cognitive function in controls, the variance in the patient group could not be explained by this model. Rather, it fitted a model with a single factor representing IQ, working memory, planning, set-shifting and verbal learning. This finding is in agreement with large studies of first-episode (Keefe et al. Reference Keefe, Seidman, Christensen, Hamer, Sharma, Sitskoorn, Lewine, Yurgelun-Todd, Gur, Tohen, Tollefson, Sanger and Lieberman2004; Addington et al. Reference Addington, Saeedi and Addington2005) and established schizophrenia (Keefe et al. Reference Keefe, Bilder, Harvey, Davis, Palmer, Gold, Meltzer, Green, Miller, Canive, Adler, Manschreck, Swartz, Rosenheck, Perkins, Walker, Stroup, McEvoy and Lieberman2006), which found that all cognitive scores loaded on a single factor. Dickinson et al. (Reference Dickinson, Ragland, Calkins, Gold and Gur2006), in a factor analysis study, also found that more of the variance in observed cognitive performance was determined by generalized cognitive ability in schizophrenia compared to healthy controls.
As verbal memory retention emerged as an independent measure in the patient group, we examined whether two dimensions of cognitive impairment, generalized deficit and impaired verbal memory retention, could independently distinguish patients and controls. In a logistic model predicting group, we found that when all variables contributing to the general factor were entered in a single block, this was a strong predictor of group membership, but when the verbal memory retention score was entered subsequently, this was still able to significantly predict group membership. Thus, the variation in verbal memory retention scores that distinguished the patients and controls seemed to be independent of the variation in the other test scores. This supports the view that this is an independent deficit. A recent factor analysis study (Dickinson et al. Reference Dickinson, Ragland, Gold and Gur2008) is consistent with our findings. This examined the factor structure of cognition in patients with schizophrenia and healthy controls and, although there was a generalized cognitive deficit across all domains in the patient group, there remained direct effects of diagnosis on verbal memory and processing speed. The authors concluded that these aspects of neurocognitive functioning may be ‘more specifically implicated in schizophrenia than other cognitive domains’.
Our finding supports the view that IQ and executive impairments in schizophrenia reflect a common abnormality of information processing rather than a collection of specific impairments (Dickinson et al. Reference Dickinson, Ramsey and Gold2007) and that this includes verbal learning but not verbal memory retention. In turn, this implies that many different forms of cognitive impairment share the same abnormal neural underpinnings and/or aetiological factors. Verbal memory retention, being different in this respect, may have a more distinct aetiology, such as increased vulnerability to environmental influences. There is some support for this conjecture. A recent study examined performance on the Weschler Logical Memory Scale in schizophrenia patients, sibling and controls (Skelley et al. Reference Skelley, Goldberg, Egan, Weinberger and Gold2008) and found that, whereas both patients and siblings were impaired on immediate and 30-min delayed recall, only patients demonstrated attenuated savings scores.
Epidemiological evidence suggests that poor verbal memory is associated with an earlier age of onset of psychosis, independent of a family loading of psychosis (Tuulio-Henriksson et al. Reference Tuulio-Henriksson, Partonen, Suvisaari, Haukka and Lonnqvist2004), indicating that verbal memory impairment might reflect an environmentally mediated risk factor for an earlier onset (see Joyce, Reference Joyce2005). Structural magnetic resonance imaging (MRI) studies of schizophrenia find that the most striking and consistent brain volume reductions are in the left MTL (Wright et al. Reference Wright, Rabe-Hesketh, Woodruff, David, Murray and Bullmore2000; Honea et al. Reference Honea, Crow, Passingham and Mackay2005). This observation may be relevant to the finding that foetal hypoxia, known to have a neurotoxic effect on the hippocampus, is also a risk factor for an earlier onset of psychosis (Cannon et al. Reference Cannon, Rosso, Hollister, Bearden, Sanchez and Hadley2000; Rosso et al. Reference Rosso, Cannon, Huttunen, Huttunen, Lonnqvist and Gasperoni2000). Finally of interest is the syndrome of ‘developmental amnesia’, which occurs following a hypoxic insult at birth or in early childhood and is characterized by isolated hippocampal pathology, impaired episodic memory and relatively preserved recognition memory and judgement of familiarity (Vargha-Khadem et al. Reference Vargha-Khadem, Gadian and Mishkin2001). Although the delayed recall deficit is much more severe in this disorder than in schizophrenia, it has a similar pattern of verbal memory impairment and illustrates how an environmental insult in early life can be associated with specific verbal memory impairments later in life, and thus may be a partial model of one aspect of the cognitive impairment in schizophrenia.
Acknowledgements
This study was funded by a programme grant from the Wellcome Trust (number 064607). E.M.J. is supported by The Raymond Way Fund. We are grateful to the consultants and nurses of West London and South West London and St George's Mental Health National Health Service (NHS) Trusts for greatly facilitating the study.
Declaration of Interest
T.R.E.B. has acted as a consultant for Servier, Johnson & Johnson and Bristol–Myers Squibb in the past 3 years. T.W.R. is a consultant for Cambridge Cognition.






