Introduction
Individuals suffering from major depressive disorder (MDD) process information about the self, the world and the future in a maladaptive fashion compared with healthy individuals (APA, 2000). According to prominent cognitive theories of depression, such as Beck's cognitive model (Beck et al. Reference Beck, Rush, Shaw and Emery1979; Haaga & Beck, Reference Haaga and Beck1995; Disner et al. Reference Disner, Beevers, Haigh and Beck2011), Seligman's learned helplessness model (Seligman, Reference Seligman1972) and the more recent cognitive neuropsychological model (Clark et al. Reference Clark, Chamberlain and Sahakian2009; Roiser et al. Reference Roiser, Elliott and Sahakian2012), maladaptive cognitive biases are central to the development and maintenance of MDD. Beck's cognitive model, for example, emphasizes the role of maladaptive cognitive schemas and has led to the development of cognitive therapy, an effective treatment focused on changing these maladaptive cognitive schemas (Beck, Reference Beck2005; Beck & Dozois, Reference Beck and Dozois2011). Related neuropsychological models of depression emphasize the relationship between maladaptive cognition and vulnerability or resilience to MDD, highlighting that maladaptive cognition may be causal in the progression of depressive symptoms (Clark et al. Reference Clark, Chamberlain and Sahakian2009; Roiser et al. Reference Roiser, Elliott and Sahakian2012).
Cognitive theories of MDD often highlight that maladaptive cognition manifests as negativity biases (e.g. Beck et al. Reference Beck, Rush, Shaw and Emery1979). However, in some instances the behavior of depressed individuals seems to be better characterized by realism relative to an objective standard or by an absence of a positivity bias relative to healthy individuals (Alloy & Ahrens, Reference Alloy and Ahrens1987; Moore & Fresco, Reference Moore and Fresco2012). The general reasoning that MDD may be related to a reduction, absence or reversal of positivity biases relative to mental health is motivated by considerable evidence showing that healthy individuals are characterized by a diverse array of positivity biases including illusions of superiority (Taylor & Brown, Reference Taylor and Brown1988; Taylor & Brown, Reference Taylor and Brown1994; Leary, Reference Leary2007), illusions of control (Taylor & Brown, Reference Taylor and Brown1988; Thompson et al. Reference Thompson, Armstrong and Thomas1998), positivity biases in memory (Walker et al. Reference Walker, Skowronski and Thompson2003) and also unrealistic optimism about the future (Weinstein, Reference Weinstein1980; Taylor & Brown, Reference Taylor and Brown1988; Weinstein & Klein, Reference Weinstein and Klein1995; Armor & Taylor, Reference Armor, Taylor, Gilovich, Griffin and Kahneman2002; Puri & Robinson, Reference Puri and Robinson2007; Sharot, Reference Sharot2011). However, the precise relationship between mental health, MDD and cognitive information processing biases (or their absence) has remained surprisingly underexplored.
So far, information processing in MDD patients has been mostly investigated in tasks that are not directly related to positivity biases in healthy individuals (Mathews & MacLeod, Reference Mathews and MacLeod2005; Gotlib & Joorman, Reference Gotlib and Joormann2010). For example, depressed individuals show altered responses to performance feedback in cognitive tasks such as the Tower of London planning task (Elliot et al. Reference Elliott, Baker, Rogers, O'Leary, Paykel, Frith, Dolan and Sahakian1997, Reference Elliott, Sahakian, Michael, Paykel and Dolan1998) or reversal learning tasks (Murphy et al. Reference Murphy, Michael, Robbins and Sahakian2003; Robinson et al. Reference Robinson, Cools, Carlisi, Sahakian and Drevets2012; see Eshel & Roiser, Reference Eshel and Roiser2010 for review). Furthermore, depressed individuals show altered reward processing as demonstrated by signal-detection analyses (Pizzagalli et al. Reference Pizzagalli, Jahn and O'Shea2005, Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava2008), computational reinforcement learning approaches (Huys et al. Reference Huys, Vogelstein, Dayan, Koller, Schuurmans, Bengio and Bottou2009) and functional neuroimaging (Tremblay et al. Reference Tremblay, Naranjo, Graham, Herrmann, Mayberg, Hevenor and Busto2005; Steele et al. Reference Steele, Kumar and Ebmeier2007; Eshel & Roiser, Reference Eshel and Roiser2010). These studies provide considerably evidence for altered learning and information processing in MDD and discuss whether MDD is better characterized by negative biases (i.e. increased responses to negative compared with positive stimuli) or by a blunting of responses (i.e. an insensitivity to both negative and positive stimuli) (Eshel & Roiser, Reference Eshel and Roiser2010; Gotlib & Joorman, Reference Gotlib and Joormann2010). However, studies on information processing in MMD typically do not investigate domains in which healthy individuals tend to show positivity biases.
In the current study, we aimed at investigating cognitive biases in processing information about future life events. In contrast to healthy individuals (Sharot, Reference Sharot2011), MDD patients show a pervasive pessimism about their personal future (APA, 2000). For example, when estimating the likelihood of experiencing positive and negative everyday life events (e.g. being invited to a party or getting a parking ticket) within the next month, individuals with high depressive symptoms expected less positive and more negative events than they eventually experienced whereas healthy participants showed the opposite pattern (Strunk et al. Reference Strunk, Lopez and DeRubeis2006; Strunk & Adler, Reference Strunk and Adler2009). Thus, previous studies have highlighted that depressed individuals are pessimistic when predicting their personal future (Cropley & MacLeod, Reference Cropley and MacLeod2003; Strunk et al. Reference Strunk, Lopez and DeRubeis2006; Strunk & Adler, Reference Strunk and Adler2009) but they do not address how information that challenges these views is incorporated into existing beliefs.
We have recently shown that healthy individuals maintain optimistic expectations as a result of selective updating; that is they process information about their personal future in a positively biased way (Sharot et al. Reference Sharot, Korn and Dolan2011, Reference Sharot, Guitart-Masip, Korn, Chowdhury and Dolan2012a ,Reference Sharot, Kanai, Marston, Korn, Rees and Dolan b ). Specifically, when healthy individuals estimated the probability of experiencing various adverse life events (e.g. robbery, Alzheimer's disease) and subsequently received information about how likely these events are to occur to persons living in the same sociocultural environment, they updated their beliefs more in response to desirable information that enforced optimism than to undesirable information that enforced pessimism. That is, participants changed their estimates more when the probabilities of the adverse events were lower than expected compared with when they were higher than expected, indicating a striking asymmetry in belief updating.
In the current study we tested the hypothesis that, unlike healthy individuals, depressed individuals are characterized by a breakdown of a selective updating bias in response to information about their future. To that end, we recruited healthy and MDD participants and quantified their belief changes in response to receipt of both desirable and undesirable information about future life events.
Method
Participants
Participants were recruited by flyers at the Freie Universität Berlin and from patients at the Charité–Universitätsmedizin Berlin and the Schlosspark-Klinik Berlin. Participants were assessed for psychiatric disorders using a structured clinical interview (SCID-I; Wittchen et al. Reference Wittchen, Zaudig and Fydrich1997) by a cognitive neuroscientist (C.W.K.), who had been trained by a psychotherapist in conducting the SCID-I. For four in-patients an assessment provided by the referring clinical psychiatrist was used instead of the SCID-I. Participants completed the Beck Depression Inventory (BDI; Hautzinger et al. Reference Hautzinger, Bailer and Worall1994). To relate task-related variables to participants' trait optimism, they also completed the Life Orientation Test – Revised (LOT-R; Scheier et al. Reference Scheier, Carver and Bridges1994). To ensure that healthy controls and MDD patients were matched on IQ, all participants completed a test of verbal IQ (the Wortschatztest, WST, a vocabulary test implemented in the HAWIE-R, the German adaptation of the Wechsler Adult Intelligence Scale; Schmidt & Metzler, Reference Schmidt and Metzler1992). Participants with a diagnosis of MDD were assessed on the 21-item Hamilton Depression Rating Scale (HAMD-21; Hamilton, Reference Hamilton1960) by a cognitive neuroscientist (C.W.K.), who had been trained by a psychotherapist in assessing the HAMD-21. As patients were only assessed by one person, inter-rater reliabilities could not be calculated. Additionally, to assess possible influences of participants' mood state on our task, they completed a multidimensional mood state questionnaire at the beginning and at the end of the study (Mehrdimensionaler Befindlichkeitsfragebogen, MDBF; Steyer et al. Reference Steyer, Schwenkmezger, Notz and Eid1997). Participants gave informed consent and were paid. The study was approved by the Ethics Committee of the Charité – Universitätsmedizin Berlin.
We recruited two groups of participants: the healthy control group (n = 19) included participants with no psychiatric disorders according to SCID-I, a BDI score ≤ 10 and no history of MDD. The MDD patients group (n = 19) included participants with a diagnosis of MDD. One MDD patient was excluded because of a history of alcoholism (final n = 18). Co-morbid anxiety disorders in the MDD group were not excluded (n = 8). Of the 18 MDD patients, six received a single drug and seven two or more (eight took selective serotonin reuptake inhibitors, four selective serotonin and noradrenaline reuptake inhibitors, three bupropion, two tricyclic antidepressants, two pregabalin, one melperone, one lorazepam and one promethazine). Table 1 shows the demographics and clinical characteristics of the participants and their scores on the mood state questionnaire.
Table 1. Demographic and clinical characteristics of the participants
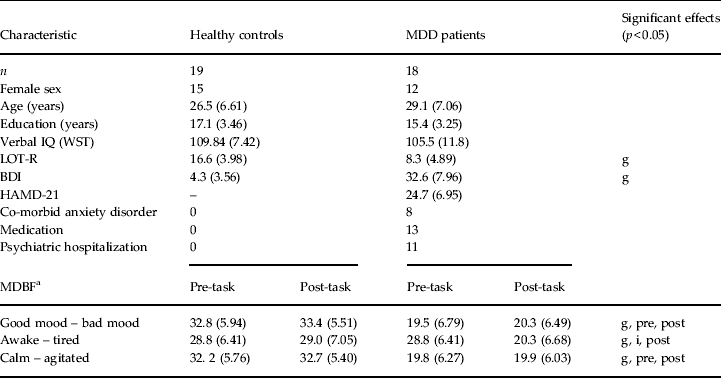
MDD, Major depressive disorder; WST, Wortschatztest, a vocabulary test implemented in the HAWIE-R (German adaptation of the Wechsler Adult Intelligence Scale); LOT-R, Life Orientation Test – Revised; BDI, Beck Depression Inventory; HAMD-21, 21-item Hamilton Depression Rating Scale; MDBF, Multidimensional Mood State Questionnaire (Mehrdimensionaler Befindlichkeitsfragebogen); g, significant group difference or significant main effect: group (p < 0.05); i, significant interaction between time and group (p < 0.05); pre, significant difference between group in pre-task condition (p < 0.05); post, significant difference between group in post-task condition (p < 0.05).
Data are given as mean (standard deviation) or number of participants.
a Data from two healthy controls were not collected post-task on the MDBF.
Stimuli
Stimuli and task were adapted from our previous studies (Sharot et al. Reference Sharot, Korn and Dolan2011, Reference Sharot, Guitart-Masip, Korn, Chowdhury and Dolan2012a ,Reference Sharot, Kanai, Marston, Korn, Rees and Dolan b ). The original English task and stimuli were translated into German by a native German speaker with English as a second language (C.W.K.). Seventy short descriptions of negative life events were used (e.g. Alzheimer's disease, robbery; see Table 2 for a complete list of the original English and the translated German stimuli). For each adverse life event, the average probability or frequency of that event occurring at least once to a person living in the same sociocultural environment as the participants was determined based on online resources (e.g. Office for National Statistics, Eurostat, PubMed). As the probabilities of the events are roughly the same across Western Europe, we used the original event probabilities (from the UK). Participants were told that they would see the probability of the event happening to an average person of a similar background living in the same place. None of the participants reported doubts about this statistical information. Very rare and very common events were not included; that is, all event probabilities lay between 10% and 70%. To ensure that the range of possible overestimation was equal to the range of possible underestimation, participants were told that the range of probabilities lay between 3% and 77%. We excluded life events that are clearly related to depressive symptoms such as severe insomnia or anxiety disorder.
Table 2. List of stimuli

Task
The task was programmed using the MATLAB toolbox Cogent 2000 (www.vislab.ucl.ac.uk/cogent.php). Participants completed four blocks of stimuli. The 70 adverse life events were split into two lists of 35 events each, which were matched for event probability. One list was used for blocks 1 and 2, the other for blocks 3 and 4. In blocks 1 and 3, participants first estimated their probability of encountering the events and then were presented with the average probability of the events for a demographically similar population (Fig. 1). To assess how participants used the information provided in block 1, they were asked to re-estimate their likelihood of encountering the events in block 2. Similarly, the likelihoods of events estimated in block 3 were re-estimated in block 4.

Fig. 1. Paradigm. (a) On each trial participants were presented with a short description of one of 70 adverse life events and asked to estimate how likely this event was to occur to them in their lifetime. They were then presented with the average probability of that event occurring to a person living in the same sociocultural environment. The second session was the same as the first session, except that the average probability of the event occurring was not presented again. For each event an update term was calculated as the difference between the participants' first and second estimations. (b,c) Examples of trials for which the participant's estimate was (b) higher or (c) lower than the average probability. Here, for illustration purposes, the thick black and gray frames denote the participant's response (either an overestimation or an underestimation respectively). The black and gray filled boxes denote information that calls for an adjustment in (b) a desirable (optimistic) or (c) an undesirable (pessimistic) direction.
Estimates and average frequencies were framed as either ‘happening’ or ‘not happening’ so as to exclude the possibility that the results could be attributed to different processing strategies for high or low numbers. Specifically, in half of the blocks (either blocks 1 and 2 or blocks 3 and 4) participants had to estimate the probability of adverse life events happening to them (and were presented with the probability of the event happening for a demographically similar population) whereas in the other half they estimated the probability of the events not happening to them (and were presented with the probability of the event not happening for a demographically similar population). List assignment and order of the aforementioned framing were counter-balanced across participants. Participants completed two training trials before blocks 1 and 3 to get familiar with the task and the change in framing.
On each trial, participants were presented with one of the 70 adverse life events for 4 s (Fig. 1) and were instructed to imagine that event happening to them in the future. Then they provided their estimate of how likely the event was to happen (or not to happen) to them in the future. Participants had up to 10 s to respond using the keyboard. They then saw a fixation cross for 1.2 s. In the blocks where participants indicated their first estimate (blocks 1 and 3), they were then presented with the average probability of the event happening (or not happening) for a demographically similar population for 3 s followed by a fixation cross of 1.2 s. In the blocks in which participants re-estimated their likelihood of encountering the events (blocks 2 and 4), they were not presented with the average frequencies of the events. The order of life events was randomized within each block.
Memory and subjective scales
After completing the four blocks of the main task we tested participants' memory for the information presented. Specifically, we asked them to indicate the average probability of each event happening as presented previously in blocks 1 and 3 (self-paced). Participants then rated all stimuli on six subjective Likert scales (self-paced): vividness (How vividly could you imagine this event? 1 = not vivid at all, 6 = very vivid), familiarity (Regardless if this event has happened to you before, how familiar do you feel it is to you from TV, friends, movies and so on? 1 = not familiar at all, 6 very familiar), prior experience (Has this event happened to you before? 1 = never, 6 = very often), emotional arousal (When you imagine this event happening to you how emotionally arousing is the image in your mind? 1 = not arousing at all, 6 = very arousing), negativity (How negative would this event be for you? 1 = not negative at all, 6 = very negative) and controllability (How much control do you have over this event? 1 = not at all, 6 = very much).
Data analysis
Data were analyzed using MATLAB and SPSS. All estimates and average frequencies in the ‘not happen’ sessions were transformed into the corresponding numbers of the ‘happen’ sessions by subtracting the respective number from 100. For each event an estimation error term was calculated as the difference between participants' first estimate and the corresponding average probability presented:
By this definition, estimation errors were positive for overestimations and negative for underestimations. Note that all life events were negative events and that the desirability of the information arose out of whether participants over- or underestimated the probability of the events. When participants initially overestimated the probability of the adverse event relative to the average probability they received desirable information (i.e. the negative event is less likely to happen than estimated; Fig. 1 b). By contrast, when participants underestimated the probability of the event relative to the average probability they received undesirable information (i.e. the negative event is more likely to happen than estimated; Fig. 1 c). Therefore, for each participant, trials were classified according to whether the participant initially overestimated or underestimated the probability of the event (i.e. according to whether estimation errors were positive or negative).
To assess how much participants changed their ratings after receiving information, an update term was calculated as the difference between the first and second estimates:
We expected participants to change their estimates on average towards the information presented. That is, for desirable information (overestimations) first estimates should be larger than second estimates (i.e. mean updates for desirable information should be positive). For undesirable information (underestimations) first estimates should be smaller than second estimates (i.e. mean updates for undesirable information should be negative). The crucial test for biased updating was whether participants changed their estimates numerically more (or less) towards desirable information than towards undesirable information. Therefore, we compared absolute mean updates for desirable and for undesirable information across participants. To exclude the possibility that differences in mean estimation errors across participants and conditions could account for differences in updating, we calculated scaled absolute mean update scores (i.e. we divided absolute mean update scores for each participant and condition by the respective absolute mean estimation errors).
Trials were excluded if (1) participants failed to answer within the allotted time (maximal 10 s) in the first or second session (healthy controls: mean = 1.79, s.d. = 1.18; MDD patients: mean = 1.50, s.d. = 1.29) or (2) the estimation error was zero (i.e. participants gave a first estimate of their own likelihood that was the same as the presented probability; healthy controls: mean = 0.68, s.d. = 1.20; MDD patients: mean = 1.28, s.d. = 1.78). These trials were excluded because they could not be classified as desirable or undesirable. In both cases the number of excluded trials did not differ between healthy controls and MDD patients as assessed by Mann–Whitney U tests (all p > 0.1).
To test the strength of association between estimation errors and updates, Pearson correlation coefficients were calculated separately for desirable and undesirable trials within each participant.
Memory errors were calculated as the absolute differences between the frequencies previously presented and participants' recollection of these statistical numbers:
Unless specified otherwise, we conducted desirability (desirable/undesirable) by group (MDD/healthy) ANOVAs.
Results
Groups did not differ with regard to sex, age, education and verbal IQ, as confirmed by independent t tests, Mann–Whitney U tests or χ 2 tests as appropriate (all p > 0.1; Table 1). MDD patients showed lower scores of trait optimism on the LOT-R compared with healthy controls (t 35 = 5.6, p < 0.001; Table 1). MDD patients also reported having more negative mood, and being more tired and more agitated as assessed by significant main effects of group in group (MDD/healthy) by time (pre-task/post-task) ANOVAs of the MDBF scores (Table 1). Additionally, there was a significant interaction of group and time on the subscale ‘awake-tired’ of the MDBF: pre-task the groups did not differ but post-task MDD patients were more tired than healthy controls.
Comparison of updating behavior between MDD patients and healthy controls
Our main hypothesis was that belief updating behavior would differ between MDD patients and healthy controls. We predicted healthy controls would show optimistically biased updating (i.e. we expected them to update their beliefs more in response to desirable than in response to undesirable information regarding adverse life events) and that this optimistic bias would be reduced, absent or reversed in MDD patients. In line with our hypothesis, there was a significant desirability (desirable/undesirable) by group (MDD/healthy) interaction of absolute mean update scores (F 1,35 = 6.9, p = 0.013, η 2 p = 0.17; Fig. 2 a; Table 3). This interaction was characterized by an asymmetry in belief updating for healthy participants but not for MDD patients. Specifically, healthy participants updated their beliefs to a greater extent in response to desirable, compared with undesirable, information (t 18 = 3.0, p = 0.008). No difference in updating was evident between desirable and undesirable trials in MDD patients (t 17 = –0.11, p > 0.9). The significant interaction was further characterized by reduced updating in response to desirable information of MDD patients relative to healthy controls (t 35 = –2.2, p = 0.033), with no significant difference for updating in response to undesirable information (t 35 = 1.4, p > 0.1). The main effect of desirability was significant (F 1,35 = 7.4, p = 0.010, η p 2 = 0.17). The main effect of group was not significant (F 1,35 = 0.73, p > 0.3, η p 2 = 0.02).
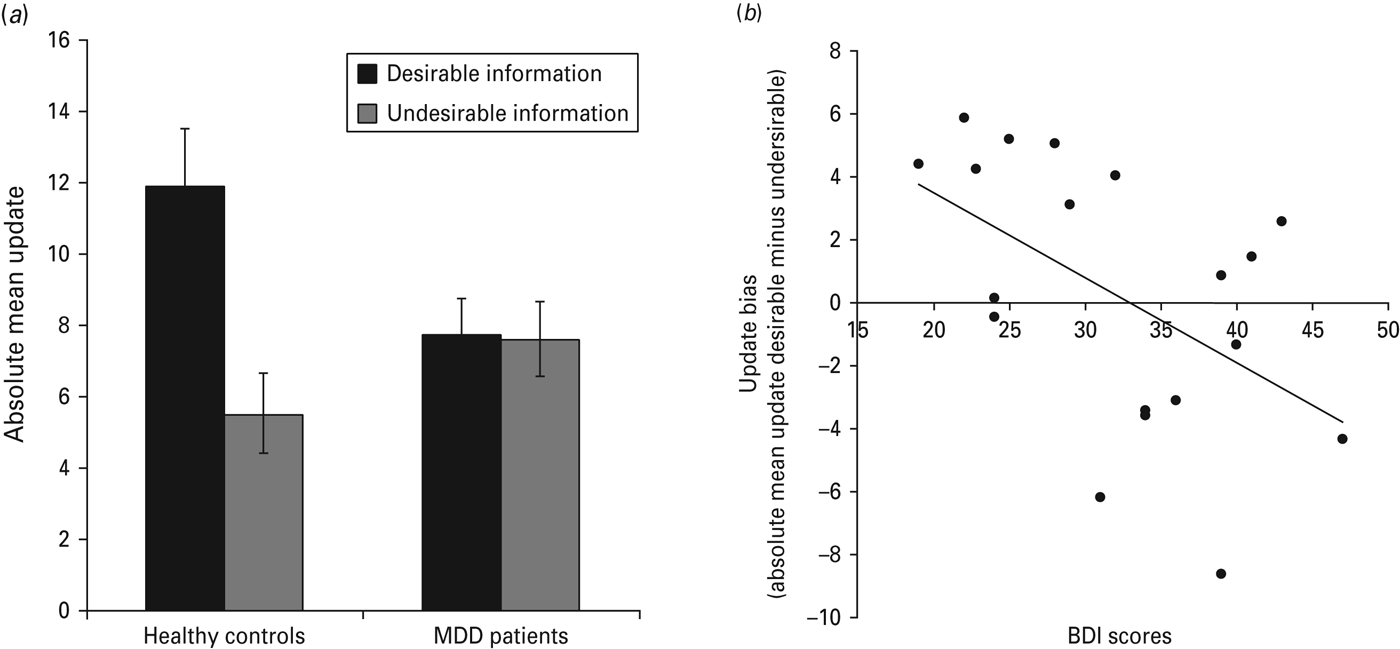
Fig. 2. Updating behavior. (a) In the healthy group absolute mean updates were greater on trials where participants received desirable information than on trials where they received undesirable information. This bias was absent in the major depressive disorder (MDD) group. (b) Relationship between Beck Depression Inventory (BDI) scores and update bias (desirable minus undesirable) in MDD patients. Error bars indicate standard error of the mean.
Table 3. Task-related variables, subjective scales, memory and reaction times
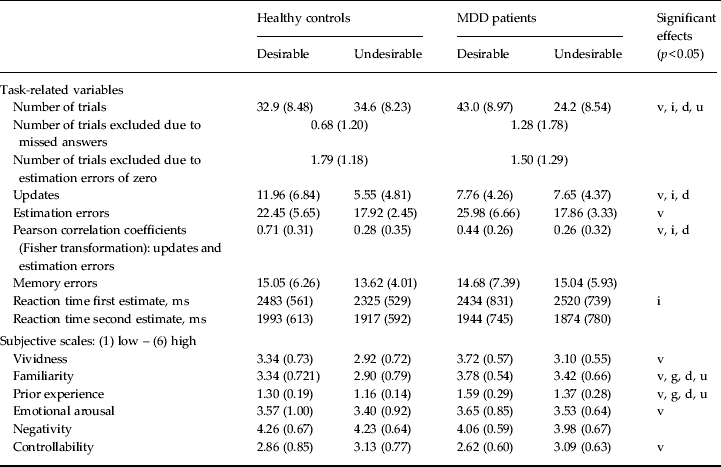
MDD, Major depressive disorder; v, significant main effect: valence (p < 0.05); g, significant main effect: group (p < 0.05); i, significant interaction between valence and group (p < 0.05); d, significant difference between group in desirable condition (p < 0.05); u, significant difference between group in undesirable condition (p < 0.05).
Data are given as mean (standard deviation).
Additional analyses: updating behavior
To exclude the possibility that the observed difference in updating between MDD patients and healthy controls was driven by differences in estimation errors (first estimations minus probabilities presented), we performed an additional ANOVA on scaled absolute mean update scores (i.e. we accounted for differences in mean estimation errors by dividing absolute mean update scores for each participant and condition by the respective absolute mean estimation errors). The desirability by group interaction of scaled absolute mean update scores was significant (interaction desirability by group: F 1,35 = 6.0, p = 0.020, η p 2 = 0.15; main effect desirability: F 1,35 = 0.67, p > 0.4, η p 2 = 0.02; main effect group: F 1,35 = 0.53, p > 0.4).
In our previous study on healthy participants, we analyzed the association between estimation errors and updates because formal learning models suggest that updates rely on error signals (i.e. the differences between expectations and outcomes) (Sharot et al. Reference Sharot, Korn and Dolan2011). The strength of the association between estimation errors and updates is indicative of an optimistic bias in healthy individuals. Specifically, for desirable information estimation errors are more closely tied to updates than for undesirable information. Given these previous results in healthy individuals, we expected, as for updates, a desirability by group interaction of the strength of the association between estimation errors and updates. For each participant we calculated the correlation between estimation errors and updates separately for desirable and undesirable trials. There was a significant desirability by group interaction (mean Fisher-transformed Pearson correlation coefficients revealed an interaction of desirability by group: F 1,35 = 4.19, p = 0.048, η p 2 = 0.11; main effect desirability: F 1,35 = 26.9, p = 0.001, η p 2 = 0.43; main effect group: F 1,35 = 2.96, p = 0.094, η p 2 = 0.08; Table 3). This further suggests that belief updating shows a less optimistic pattern in MDD patients compared with healthy controls.
Relationship between differential updating and MDD symptoms
Next, we sought to test whether the update bias (i.e. the difference between updates for desirable versus undesirable information) was related to depressive symptoms as measured by the BDI (Fig. 2 b). BDI scores correlated negatively with the update bias across MDD patients (Pearson's r = –0.50, p = 0.036) but no significant correlation emerged for healthy controls (r = –0.001, p = 0.996), for which the range of BDI scores was limited. Thus, MDD patients with more severe symptoms showed less optimistic updating and more pessimistic updating (i.e. updating more in response to undesirable information compared with desirable information).
To elucidate whether group differences in update bias are potentially independent from depression severity, we tested whether group differences remained when partialling out BDI scores. We conducted a hierarchical regression analysis across all participants entering update bias as the dependent variable. As independent variables, we entered BDI scores on the first level and group membership on the second. BDI scores significantly predicted update bias (F 1,35 = 8.41, p = 0.006) but group membership explained no additional variance beyond BDI scores [F change(1,34) = 0.00, p > 0.9], suggesting that differential updating is not independent of depression severity.
Additional analyses: relationship between differential updating and participants' characteristics and task-related variables
To test whether the update bias was related to demographic characteristics, mood states, task-related variables or subjective scales, we conducted a stepwise linear regression analysis entering update bias as the dependent variable. As independent variables we entered group membership, BDI, and their interaction, along with age, gender, education, presence of co-morbid anxiety disorder, medication status, verbal IQ, LOT-R scores, initial mood scores (Table 1) and differential measures (desirable minus undesirable) of memory errors, reaction times, estimation errors, and also differential scores on all subjective scales (vividness, familiarity, prior experience, arousal, negativity, controllability; Table 3). The model that best predicted differential updating only included the interaction of group membership with BDI score (F 1,35 = 8.60, p = 0.006). Demographic characteristics, mood states, task-related variables or subjective scales were not retained in the stepwise regression. That is, the update bias was influenced by whether participants were healthy or depressed and the BDI score predicted the update bias in MDD patients.
In addition, we specifically analyzed the relationship between update bias and LOT-R scores. In MDD patients, LOT-R scores correlated with the update bias (r = –0.52, p = 0.027) but they did not explain additional variance beyond the BDI in a hierarchical regression [F change(1,15) = 1.107, p > 0.3]. In healthy controls, LOT-R scores did not correlate significantly with the update bias (r = –0.19, p > 0.4).
Additional analyses: comparison of initial estimates between MDD patients and healthy controls
In accord with the general pessimistic tendency of MDD patients, MDD patients initially estimated their overall probability of experiencing adverse life events as greater than healthy controls (MDD patients: first estimate = 39.9, s.d. = 8.20; healthy controls: first estimate = 31.5, s.d. = 7.47; t 35 = 3.3, p = 0.002). MDD patients overestimated their probability of experiencing negative life events in relation to the average frequencies for a demographically similar population; that is mean estimation errors (first estimation minus probability presented) were positive and significantly different from zero (estimation error: mean = 10.4, s.d. = 8.18 ; t 17 = 5.4, p < 0.001). This was not the case for healthy controls (estimation error: mean = 1.80, s.d. = 7.40; t 18 = 1.1, p > 0.3). Mean estimation errors differed between groups (t 35 = 3.3, p = 0.002).
As MDD patients were more pessimistic overall compared with healthy controls, the number of overestimations (desirable trials) and the number of underestimations (undesirable trials) differed between the two groups. Specifically, there was a significant desirability (desirable/undesirable) by group interaction of the number of trials (interaction desirability by group: F 1,35 = 13.6, p < 0.001, η p 2 = 0.28; main effect desirability: F 1,35 = 9.40, p = 0.004, η p 2 = 0.21; Table 3). For MDD patients the number of trials with desirable information was greater than the number of trials with undesirable information (t 17 = 4.6, p < 0.001) because they overestimated the probabilities more often than they underestimated them. For healthy controls the number of trials with desirable and undesirable information did not differ (t 18 = –0.46, p > 0.6). Furthermore, the interaction was characterized by MDD patients receiving desirable information on more trials and undesirable information on less trials than healthy controls (desirable: t 35 = 3.54, p = 0.001; undesirable: t 35 = –3.80, p < 0.001). There was no group difference in the overall number of trials (main effect group: F 1,35 = 0.25, p > 0.6, η p 2 = 0.01).
Taken together, even though MDD patients received more desirable information than healthy individuals (and thus had more opportunities to change their beliefs in an optimistic direction), MDD patients showed no evidence for optimistically biased updating.
Additional analyses: memory, subjective rating scales, framing and reaction times
Differences in updating are not explained by differences in memory for the presented probability, by differences in subjective ratings of events, or by differences related to the framing of the presented probability. Specifically, we asked participants to recollect the presented probability of the event happening and computed memory errors for each event as the absolute differences between participants' recollection and the probabilities presented. There was no significant desirability by group interaction for absolute memory errors (interaction desirability by group: F 1,35 = 1.46, p > 0.2, η p 2 = 0.04; main effect desirability: F 1,35 = 0.52, p > 0.4, η p 2 = 0.02; main effect group: F 1,35 = 0.08, p > 0.7, η p 2 = 0.00; Table 3). Additionally, we asked participants to rate all negative events on six scales for vividness, familiarity, prior experience, emotional arousal, negativity and controllability. There was no significant desirability by group interaction of any of these measures (all p > 0.1; see Table 3 for significant main effects).
To control for framing effects (effects due to information being presented in a positive or negative context) we asked participants to estimate how likely the events were to happen on half of the trials and how likely they were not to happen on the other half of the trials. In a frame (happen/not happen) by desirability by group ANOVA, only the desirability by group interaction reached significance (F 1,34 = 5.74, p = 0.022, η p 2 = 0.14; all other main effects and interactions: p > 0.1; one healthy participant had to be excluded from this analysis because there were no trials in the desirable-happen condition). Thus, the framing of the estimates had no effect on updating behavior.
Reaction times for the first estimate did not show significant main effects of desirability or group (all p > 0.1) but a significant interaction (F 1,35 = 5.98, p = 0.020, η p 2 = 0.15; Table 3). The interaction was characterized by healthy controls being slower for estimates for which they subsequently received desirable information compared with estimates for which they subsequently received undesirable information (t 18 = –0.46, p = 0.006). This was not the case for MDD patients (p > 0.3). Reaction times for the second estimate showed no significant main effects or interaction (all p > 0.5; Table 3).
Nevertheless, differential measures (desirable minus undesirable) of memory errors, scores on all subjective scales and reaction times were included in the stepwise linear regression analysis described earlier, which revealed that only the interaction of group membership with the BDI score was retained in the model that best predicted differential updating.
Discussion
We show an absence of optimistic bias in belief updating in depressed individuals and this absence correlated with their symptom severity. Healthy individuals updated their beliefs more when presented with desirable information about the likelihood of experiencing adverse life events relative to undesirable information. By contrast, updating from desirable versus undesirable information correlated with symptom severity in MDD patients: less severely depressed individuals showed a positive bias but more severely depressed individuals showed a negative bias (i.e. they update more from undesirable compared with desirable information). Overall, this resulted in an absence of updating asymmetry across our sample of depressed individuals. Note that both groups were responsive to the information presented in the task and updated their estimates accordingly. The key difference between the groups was that, whereas controls showed a valence-dependent updating bias, the MDD group on average showed an absence of this bias. This lack of biased updating in MDD patients was due to reduced updating from desirable information about the future.
The observed pattern was selective for updating behavior (i.e. memory for the information presented and subjective ratings of the events did not show interactions between the two groups and the desirability of the information). The current results in healthy individuals replicate our previous findings (Sharot et al. Reference Sharot, Korn and Dolan2011, Reference Sharot, Guitart-Masip, Korn, Chowdhury and Dolan2012a ,b) in a German sample and are in line with studies demonstrating that healthy individuals see emotionally laden future events through rose-colored spectacles (Sharot et al. Reference Sharot, Riccardi, Raio and Phelps2007; Szpunar et al. Reference Szpunar, Addis and Schacter2012).
In accord with cognitive theories of depression (e.g. Beck et al. Reference Beck, Rush, Shaw and Emery1979), depressed individuals exhibited a pessimistic view of the future, evident in their inflated estimates of the probabilities of experiencing adverse events relative to controls and to the average probabilities of these events in the population. These results are also in line with previous studies (Strunk et al. Reference Strunk, Lopez and DeRubeis2006; Strunk & Adler, Reference Strunk and Adler2009) showing that depressed individuals expect more negative events and less positive events within the upcoming month than healthy controls. In our task, MDD patients received more desirable and less undesirable information because of their pessimistic views. Nevertheless, in contrast to controls, MDD patients did not take desirable information more into account than undesirable information but showed an absence of optimistically biased updating despite receiving more information that would warrant such an optimistic bias. It is possible that the more optimistic expectations of healthy compared with depressed individuals are a result of increased responsiveness to desirable information relative to negative information regarding the future; although cause and effect may also be reversed. Taken together, the current study showed that depressed individuals were characterized by pessimistic expectations and the absence of an optimistic updating bias.
Compared to many previous studies that have shown evidence for altered learning and feedback processing (Gotlib & Joorman, Reference Gotlib and Joormann2010; Eshel & Roiser, Reference Eshel and Roiser2010), our study is more directly related to the research on positivity biases in healthy individuals. That is, participants in our study received explicit information about the personal probability of future life events whereas participants in previous studies typically received outcomes in the form of performance feedback, reward or punishment (e.g. Steele et al. Reference Steele, Kumar and Ebmeier2007; Huys et al. Reference Huys, Vogelstein, Dayan, Koller, Schuurmans, Bengio and Bottou2009; Chase et al. Reference Chase, Frank, Michael, Bullmore, Sahakian and Robbins2010; Robinson et al. Reference Robinson, Cools, Carlisi, Sahakian and Drevets2012). Using information about future life events, we show that the updating behavior of the MDD patients was less responsive to desirable information relative to controls, but similarly responsive to undesirable information. Therefore, the updating behavior of the MDD patients in our task seems to be better described by a lack of a positivity bias than by notions of general emotional blunting, as discussed in previous studies (see Eshel & Roiser, Reference Eshel and Roiser2010).
Testing whether the updating bias shown by healthy individuals is adaptive is beyond the remit of the present study. However, the relative absence of optimistically biased updating in MDD needs to be considered in the context of previous research suggesting that positivity biases can be adaptive for mental and physical health and also economic success (Taylor & Brown, Reference Taylor and Brown1988, Reference Taylor and Brown1994; Scheier & Carver, Reference Scheier and Carver1992; Weinstein & Klein, Reference Weinstein and Klein1995; Peterson, Reference Peterson2000; Armor & Taylor, Reference Armor, Taylor, Gilovich, Griffin and Kahneman2002; Haselton & Nettle, Reference Haselton and Nettle2006; Leary, Reference Leary2007; Puri & Robinson, Reference Puri and Robinson2007; McKay & Dennett, Reference McKay and Dennett2009; Varki, Reference Varki2009; Johnson & Fowler, Reference Johnson and Fowler2011; Sharot, Reference Sharot2011). For example, all else being equal, it seems that optimists live longer, recover faster from diseases (see Rasmussen et al. Reference Rasmussen, Scheier and Greenhouse2009 for review), and earn more (Puri & Robinson, Reference Puri and Robinson2007). This needs to be weighted by evidence that extreme optimists do engage in unhealthy and risky behavior such as smoking and failing to save for retirement (Puri & Robinson, Reference Puri and Robinson2007; see Sharot, Reference Sharot2011 for review). Mild to moderate positivity biases may exert an adaptive effect in at least three ways. Positive beliefs can reduce stress and anxiety (Solberg Nes & Segerstrom, Reference Solberg Nes and Segerstrom2006). They can enhance a motivation to obtain desired goals; for example, optimists exercise more and work harder (Puri & Robinson, Reference Puri and Robinson2007; see Sharot, Reference Sharot2011 for review). Furthermore, positive beliefs enhance exploratory behavior that can enhance individual and group success (see Sharot, Reference Sharot2011 for review). In the context of MDD, recent research suggests that depressed individuals benefit from therapy approaches that focus on inducing positive biases, such as positive psychology interventions (Sin & Lyubomirsky, Reference Sin and Lyubomirsky2009) and cognitive bias modification (Hallion & Ruscio, Reference Hallion and Ruscio2011).
Future studies are needed to determine the generality of our findings in a larger sample that includes more male participants, and also to determine possible influences of medication and co-morbidity on the observed updating behavior. Importantly, we emphasize that the current study examines biased updating for negative but not for positive life events. Previous studies have shown that a similar updating bias exists in healthy individuals when they learn about positive stimuli (Eil & Rao, Reference Eil and Rao2011; Möbius et al. Reference Möbius, Niederle, Niehaus and Rosenblat2011; Korn et al. Reference Korn, Prehn, Park, Walter and Heekeren2012; Wiswall & Zafar, Reference Wiswall and Zafar2013), but whether such a lack of bias exists in MDD patients for positive events is an empirical question that needs to be tested.
The optimistic updating pattern described for healthy participants in our task might be associated with resilience to depression. Our study does not address whether altered information processing has a causal role in MDD. Longitudinal studies are needed to establish whether an absence of optimistically biased processing precedes the onset of depressive episodes and whether an increase in optimistic updating predicts treatment effects. Future studies will also be crucial in examining whether techniques that enhance updating from desirable information and/or techniques that reduce updating from undesirable information might be beneficial in the treatment of depression. Our results provide a starting point for such investigations by suggesting that an absence of optimistically biased belief updating of information regarding future life events may be relevant for mental health.
Acknowledgments
This study was supported by a Senior Investigator Award (098362/Z/12/Z) and a Max Planck Award to R.J.D. and a Wellcome Trust Career Development Fellowship to T.S. The Wellcome Trust Centre for Neuroimaging is supported by core funding from the Wellcome Trust 091593/Z/10/Z. We thank F. Bermpohl, M. Adli, P. Sterzer and R. Schneibel for help with recruiting participants.
Declaration of Interest
None.







