Introduction
The salient behavioral disturbance that characterizes anorexia nervosa (AN) is restriction of energy intake below the body's needs, leading to an underweight state. Limited caloric intake has been demonstrated via objectively measured 24-h intake (Hadigan et al., Reference Hadigan, Anderson, Miller, Hubbard, Herzog, Klibanski and Grinspoon2000), self-reported food records (Misra et al., Reference Misra, Tsai, Anderson, Hubbard, Gallagher, Soyka and Klibanski2006), and laboratory-based meals (Mayer, Schebendach, Bodell, Shingleton, & Walsh, Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012; Sysko, Walsh, Schebendach, & Wilson, Reference Sysko, Walsh, Schebendach and Wilson2005). The reduction in calorie intake is largely accomplished by individuals with AN limiting their intake of foods with high fat content (Hadigan et al., Reference Hadigan, Anderson, Miller, Hubbard, Herzog, Klibanski and Grinspoon2000; Mayer et al., Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012; Schebendach et al., Reference Schebendach, Uniacke, Walsh, Mayer, Attia and Steinglass2019). This restrictive eating pattern is linked to worse longer-term prognosis (Schebendach et al., Reference Schebendach, Mayer, Devlin, Attia, Contento, Wolf and Walsh2008; Schebendach, Mayer, Devlin, Attia, & Walsh, Reference Schebendach, Mayer, Devlin, Attia and Walsh2012). Successful short-term treatment, including restoration of body weight to normal, is, on average, not associated with improvement in what individuals choose to eat outside of supervised meals (Foerde et al., Reference Foerde, Walsh, Dalack, Daw, Shohamy and Steinglass2021; Mayer et al., Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012; Sysko et al., Reference Sysko, Walsh, Schebendach and Wilson2005). However, it is not known whether an individual's changes in food choice over the course of acute treatment impact longer-term outcome.
Eating behavior can be assessed in a number of ways, including self-report of daily intake and objective assessment in a laboratory setting. We have developed a computer-based, easily administered, Food Choice Task to capture the restricted selection of calorically dense foods in AN. In this task, participants separately rate the ‘healthiness’ and the ‘tastiness’ of 76 images of foods and then indicate to what degree they prefer each of these foods v. a reference food that they rated as having ‘neutral’ healthiness and tastiness. We have demonstrated that behavior in this task is predictive of actual food intake (Steinglass, Foerde, Kostro, Shohamy, & Walsh, Reference Steinglass, Foerde, Kostro, Shohamy and Walsh2015). Specifically, a participant's tendency to choose high-fat foods over the reference food in the task predicts their actual caloric and fat intake in a laboratory meal the following day (Foerde, Steinglass, Shohamy, & Walsh, Reference Foerde, Steinglass, Shohamy and Walsh2015). Use of the task with functional magnetic resonance imaging (fMRI) scanning has demonstrated differences in the neural circuits that individuals with AN use when deciding what foods to choose, compared to healthy controls (Foerde et al., Reference Foerde, Steinglass, Shohamy and Walsh2015, Reference Foerde, Walsh, Dalack, Daw, Shohamy and Steinglass2021, Reference Foerde, Schebendach, Davis, Daw, Walsh, Shohamy and Steinglass2022).
The Food Choice Task has also been used to quantify ‘self-control’. On this task, the need for self-control arises when healthiness and tastiness ratings are incongruent. More specifically, self-control is quantified on the task by (1) an individual's tendency to select a self-perceived ‘healthy’ item even when that item is also rated as ‘not tasty’ and (2) an individual's tendency to avoid choosing items rated as ‘tasty’ but ‘not healthy’. Among individuals without eating disorders, ratings of healthiness and tastiness are generally uncorrelated (Hare, Camerer, & Rangel, Reference Hare, Camerer and Rangel2009; Lloyd et al., Reference Lloyd, Shehzad, Schebendach, Bakkour, Xue, Assaf and Foerde2020; Maier, Raja Beharelle, Polania, Ruff, & Hare, Reference Maier, Raja Beharelle, Polania, Ruff and Hare2020) and use of self-control is aligned with health goals (Hare et al., Reference Hare, Camerer and Rangel2009).
The propensity of individuals with AN to use self-control does not align with long-term treatment goals. As foods with a high fat content are generally viewed by individuals with AN as ‘not healthy’, the tendency to avoid choosing them even when they are rated as ‘tasty’ helps perpetuate the restriction of caloric intake. That is, although the propensity to use self-control in making the choice of what to eat may support health goals among individuals without an eating disorder, for individuals with AN, the tendency to use such self-control is maladaptive. Furthermore, in contrast to their relationship in healthy individuals (Lloyd et al., Reference Lloyd, Shehzad, Schebendach, Bakkour, Xue, Assaf and Foerde2020), ratings of tastiness and healthiness are correlated among individuals with AN who tend to rate ‘not healthy’ foods as ‘not tasty’ (Foerde et al., Reference Foerde, Steinglass, Shohamy and Walsh2015, Reference Foerde, Schebendach, Davis, Daw, Walsh, Shohamy and Steinglass2022), a phenomenon that also supports persistent avoidance of high-fat foods. In the Food Choice Task, the correlation between ratings of healthiness and tastiness among individuals with AN further reduces the number of trials in which self-control can be used. Nonetheless, individuals with AN use self-control in a greater proportion of these trials than do controls, further supporting the persistent restriction of high-fat food choices (Dalton et al., Reference Dalton, Foerde, Bartholdy, McClelland, Kekic, Grycuk and Steinglass2020; Foerde et al., Reference Foerde, Steinglass, Shohamy and Walsh2015, Reference Foerde, Schebendach, Davis, Daw, Walsh, Shohamy and Steinglass2022).
Intensive treatment for AN (e.g. inpatient or day program care) is largely successful in weight restoration (Murray, Quintana, Loeb, Griffiths, & Le Grange, 2019; Zeeck et al., Reference Zeeck, Herpertz-Dahlmann, Friederich, Brockmeyer, Resmark, Hagenah and Hartmann2018). Yet, longer-term outcomes are variable. A higher body mass index (BMI) at discharge predicts better outcome at 1 year (Walsh et al., Reference Walsh, Kaplan, Attia, Olmsted, Parides, Carter and Rockert2006) and better clinical course over the 5 years after hospital discharge (Glasofer et al., Reference Glasofer, Muratore, Attia, Wu, Wang, Minkoff and Steinglass2020). In addition, patterns of food intake after weight restoration on an inpatient unit are associated with better outcome at 1 year: patients who selected a wider variety of foods and food items higher in energy density were more likely to have a good outcome (Schebendach et al., Reference Schebendach, Mayer, Devlin, Attia, Contento, Wolf and Walsh2008, Reference Schebendach, Mayer, Devlin, Attia and Walsh2012).
In the current study, we examined whether change in decisions about food choice among hospitalized patients with AN was related to clinical course over 3 years. Decisions about food were measured via the Food Choice Task and quantified by the tendency to select high-fat foods and the tendency to use self-control in food choices. We tested whether change in these task parameters over the course of inpatient treatment predicted short-term clinical outcome BMI at the time of hospital discharge and conducted a preliminary analysis of longer-term outcome (BMI and psychological features characteristic of eating disorders) during the 3 years after hospital discharge.
Methods
Participants were hospitalized individuals meeting Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013) criteria for AN and were between 14 and 60 years old. Eating disorder diagnoses were established via the Eating Disorders Assessment for DSM-5 (κ = 0.83) (Sysko et al., Reference Sysko, Glasofer, Hildebrandt, Klimek, Mitchell, Berg and Walsh2015) and confirmed by clinician interview. All participants were enrolled in studies reviewed and approved by the New York State Psychiatric Institute Institutional Review Board; informed consent (or, if under 18 years, participant assent and parental consent) was obtained prior to participation. Race, ethnicity, and sex assigned at birth were identified by self-report. Participants were included if they completed the Food Choice Task at both admission and discharge. Data were collected from patients hospitalized between March 2016 and April 2022.
Height and weight were measured upon admission, and weight was measured on the day of discharge. The Food Choice Task was administered within the first week of hospitalization, and again at the end of treatment, prior to discharge. Treatment consisted of a behaviorally based inpatient program, aiming for full weight restoration (Attia & Walsh, Reference Attia and Walsh2009). Dietary prescriptions follow a standard clinical protocol, beginning at 1800 kcal/day with regular increases to 3600 kcal in food and 1600 kcal in nutritional supplements over the first few weeks, consistent with standard guidelines. Standard nutritional counseling was provided.
On the day of task administration, patients received a standardized research lunch (~400 kcal at time 1 and ~700 at time 2) and then had nothing to eat or drink (except water) until the task was administered 2 h later. The task is incentive-compatible in that patients are informed that one of their selections during the task will be provided to them as a snack, and that item is given in lieu of their evening snack. A subset of participants (n = 26) completed the task in the Magnetic Resonance Imaging (MRI) scanner; task data from these individuals were published previously, without analyses of clinical course (Foerde et al., Reference Foerde, Walsh, Dalack, Daw, Shohamy and Steinglass2021). Admission task data from a subset of participants were published previously in an analysis of subtype (restricting v. binge-eating/purging) differences, in which groups did not differ on the task (Uniacke et al., Reference Uniacke, Slattery, Walsh, Shohamy, Foerde and Steinglass2020).
The Food Choice Task (Steinglass et al., Reference Steinglass, Foerde, Kostro, Shohamy and Walsh2015) consists of three blocks: healthiness, tastiness, and choice (Fig. 1). Photographs of 76 food items are presented in each block; 38 items are high fat (>30% total calories from fat) items and 38 are low fat. Participants are asked to rate each photograph for healthiness and tastiness on a 5-point Likert scale in two separate blocks (healthiness and tastiness). The rating scale appears at the bottom of the screen for each food and participants are instructed that they can rate it as ‘neutral’ or along the scale. For the healthiness block, the anchors are identified as ‘unhealthy’ to ‘healthy’. For the tastiness block, anchors are ‘bad’ to ‘good’. All task parameters (including order of the rating scale) are counterbalanced and randomized across participants. After healthiness and tastiness have been rated, a ‘reference’ item is selected for the individual that has been rated by them as ‘neutral’ on healthiness and tastiness. If an individual does not identify an item that is neutral on both scales, an item is selected that is neutral on healthiness and 1 point in the positive direction on tastiness to minimize biasing choices based on taste value. Participants are then presented with the choice block during which they are asked to choose between the reference item and each of the other 75 images. The reference item stays the same throughout the task. Participants are instructed that they will be served a snack-size portion of one of their choices, selected at random, as a snack that same day, enhancing confidence that responses reflect true preferences. Test–retest reliability is good to excellent (Foerde et al., Reference Foerde, Gianini, Wang, Wu, Shohamy, Walsh and Steinglass2018). The Food Choice Task is freely available for download at https://github.com/Columbia-Center-for-EDs/Food-Choice-Task.
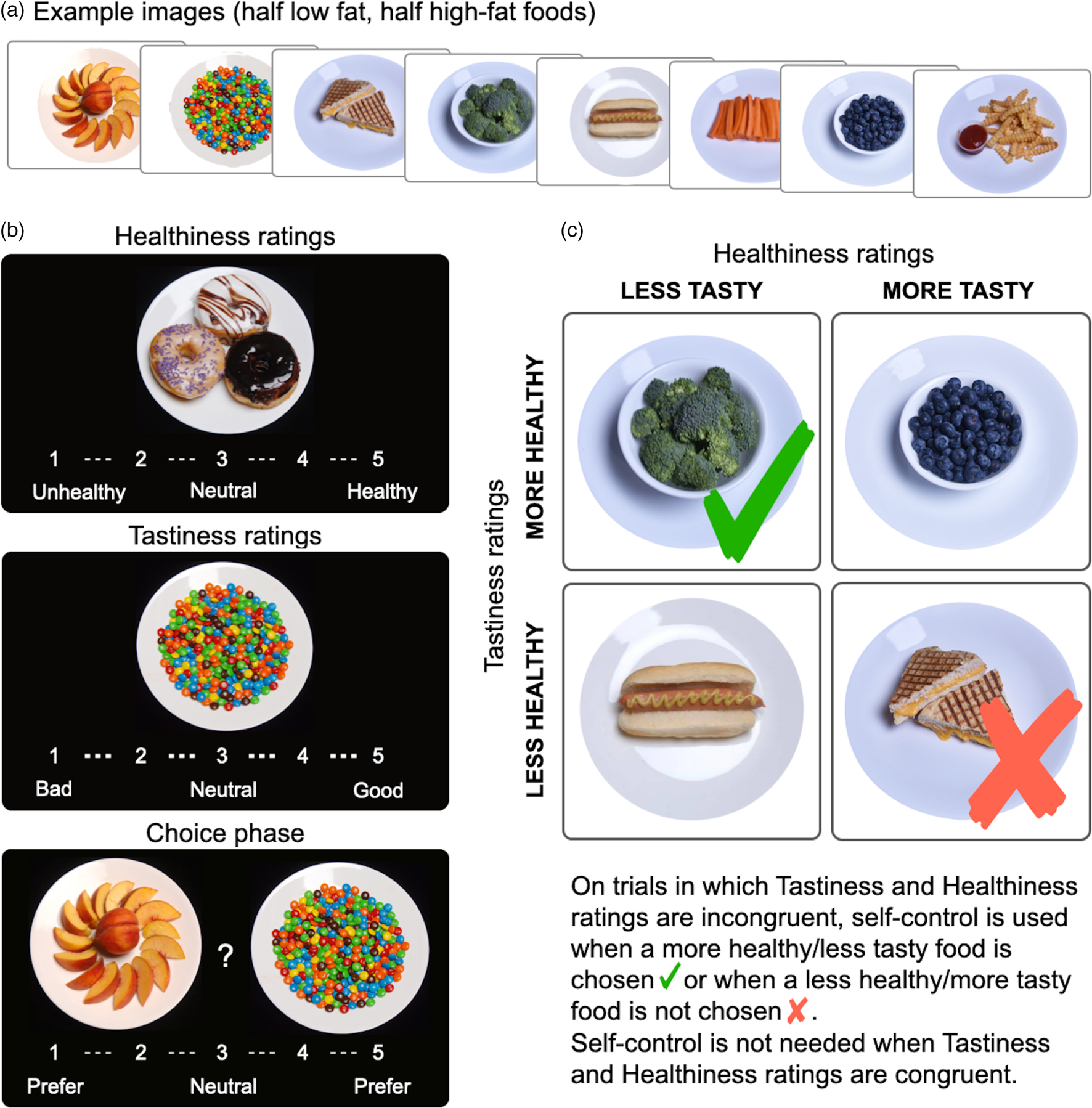
Figure 1. Food Choice Task ratings, choices and self-control. (a) Example images selected from the 76 images (38 low fat, 38 high fat) used in the Food Choice Task. (b) Task phases: healthiness and tastiness ratings (order counterbalanced) followed by choice phase, with the neutral-rated reference item presented on the left. (c) Schematic of the construction of self-controlled choices based on healthiness and tastiness ratings, with indication of choices utilizing self-control.
Longitudinal follow-up (Glasofer et al., Reference Glasofer, Muratore, Attia, Wu, Wang, Minkoff and Steinglass2020)
Annual phone interviews were conducted after hospital discharge and self-report questionnaires were distributed by survey link. Weight was obtained by self-report at each annual survey. Prior research has shown a correlation of r = 0.97, p < 0.0001 between self-reported and clinician-obtained weights in this longitudinal survey (Glasofer et al., Reference Glasofer, Muratore, Attia, Wu, Wang, Minkoff and Steinglass2020). Height from hospital stay and self-reported weight were used to calculate BMI.
The Eating Disorder Examination, Questionnaire version (EDE-Q) (Berg, Peterson, Frazier, & Crow, Reference Berg, Peterson, Frazier and Crow2012; Fairburn & Beglin, Reference Fairburn and Beglin1994) was used to assess the psychological features characteristic of AN. The EDE-Q is a self-report measure of eating disorder symptom severity over the past 4 weeks. It generates four subscale scores (restraint, eating concern, weight concern, and shape concern) and a global score (an average of the subscale scores). The EDE-Q has demonstrated internal consistency, test–retest reliability, convergent validity, and divergent validity (Berg et al., Reference Berg, Peterson, Frazier and Crow2012; Peterson et al., Reference Peterson, Crosby, Wonderlich, Joiner, Crow, Mitchell and le Grange2007). Higher scores indicate more severe symptomatology.
Clinical outcomes
Short-term clinical outcome was defined as BMI on the day of discharge. Longer-term clinical outcome was assessed via BMI and EDE-Q scores (separately) over the 3 years, using all available data (continuous outcome). Maintenance of a BMI ⩾ 18.5 kg/m2 at all available timepoints over 3 years was examined in an exploratory analysis of successful health maintenance (categorical outcome).
Data analyses
Demographics are described by means and percentages. Changes during inpatient treatment were analyzed using paired samples t tests and α was set at 0.05.
Change between admission and discharge in two parameters from the choice block was examined: (1) choice of high-fat food: the proportion of trials in which the participant chooses the high-fat food when there is a choice between a high-fat food and the reference item and (2) use of self-control: the proportion of trials in which there is an opportunity to exert self-control (i.e. the non-reference food is rated as tasty but not healthy, or not tasty but healthy) and the individual chooses a healthy, less tasty item or chooses the reference instead of an unhealthy, tasty item (see Fig. 1c). Confidence intervals (CI) are reported.
Short-term clinical status
This analysis examined the relationship between change in task parameters (proportion of high-fat foods chosen and proportion of trials using self-control) and BMI at discharge. Associations were examined using Pearson correlations and a linear regression model adjusted for covariates. For the analysis of change in choice of high-fat food, covariates included BMI at admission, age, duration of illness (in months), and diagnostic subtype (restricting or binge-eating/purging). For the analysis of change in the use of self-control, the number of trials in which self-control could have been used was included as an additional covariate. Furthermore, an interaction between diagnosis subtype and choice change was examined in each model. Association between days in treatment and change in task parameters were examined using Pearson correlation.
Longer-term clinical status
Linear mixed-effects models with subject-specific random intercepts were used to examine the relationship between change in choices on the Food Choice Task (choice of high-fat food and use of self-control) and clinical course (BMI and EDE-Q scores) for patients who participated in at least one follow-up timepoint (see Table 2). All available assessments from these patients were included in the analyses. Predictors included change in choice of high-fat food or in use of self-control (separate models), time, diagnostic subtype, age, duration of illness, BMI or EDE-Q at admission (separate models), and an interaction term between time and change in the relevant food choice variable. For analysis of change in the use of self-control, the number of trials in which self-control could have been used was also included. A categorical outcome of weight maintenance over time (BMI ⩾ 18.5 kg/m2 at all available follow-up points, regardless of BMI at discharge) was examined using a generalized linear mixed-effects model with subject-specific random intercepts; this model was repeated with health maintenance defined as BMI ⩾ 18.5 kg/m2 and EDE-Q < 2 at all available timepoints. For the model to converge, all predictors in the continuous outcome model examining the impact of change in high-fat food choice were included except duration; for the model examining the impact of change in the use of self-control, both duration and age were excluded from the model.
Results
Data were collected from 88 patients with AN who completed the Food Choice Task at admission and again at discharge. Clinical characteristics are provided in Table 1. During inpatient treatment, BMI increased significantly from 16.5 ± 2.0 kg/m2 to 20.6 ± 1.2 kg/m2 (t 87 = −24.0, p < 0.001), weight increased significantly from 96.6 ± 15.2 lbs to 120.8 ± 12.9 lbs (t 87 = −23.7, p < 0.001), and the EDE-Q score decreased significantly from 4.2 ± 1.4 to 2.8 ± 1.3 (t 80 = 10.8, p < 0.001). Eleven patients left before achieving a BMI of 19.5 kg/m2. Patients were hospitalized for a mean of 67.9 ± 27.4 days (range 21–155 days).
Table 1. Clinical characteristics
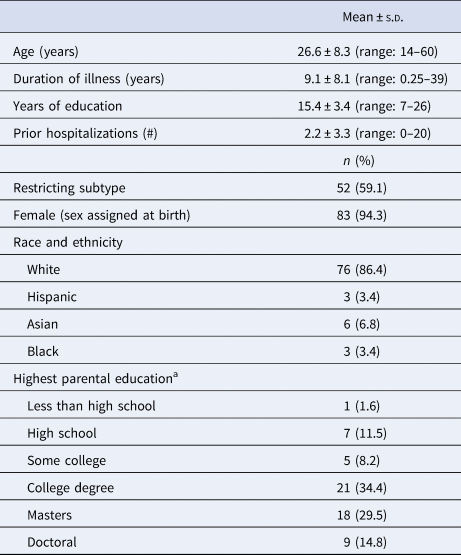
a Data are missing for 27 participants (30.1% of the sample).
Short-term clinical status
Between admission and discharge, there was no significant change in the proportion of trials in which high-fat foods were chosen over the reference food item (mean: 0.33 ± 0.26 v. 0.32 ± 0.25; t 87 = 0.40, p = 0.69; see Fig. 2a). There was also no significant change in the proportion of trials during which self-control was used (mean: 0.47 ± 0.32 v. 0.44 ± 0.34; t 81 = 0.92, p = 0.36; see Fig. 2b). Number of days in the hospital was not significantly associated with change in high-fat choices (r = −0.08, p = 0.44) nor with change in self-control (r = 0.13, p = 0.25). There was no significant association between age and change in food choice (ps > 0.6), or between duration of illness and change in food choice (ps > 0.4).
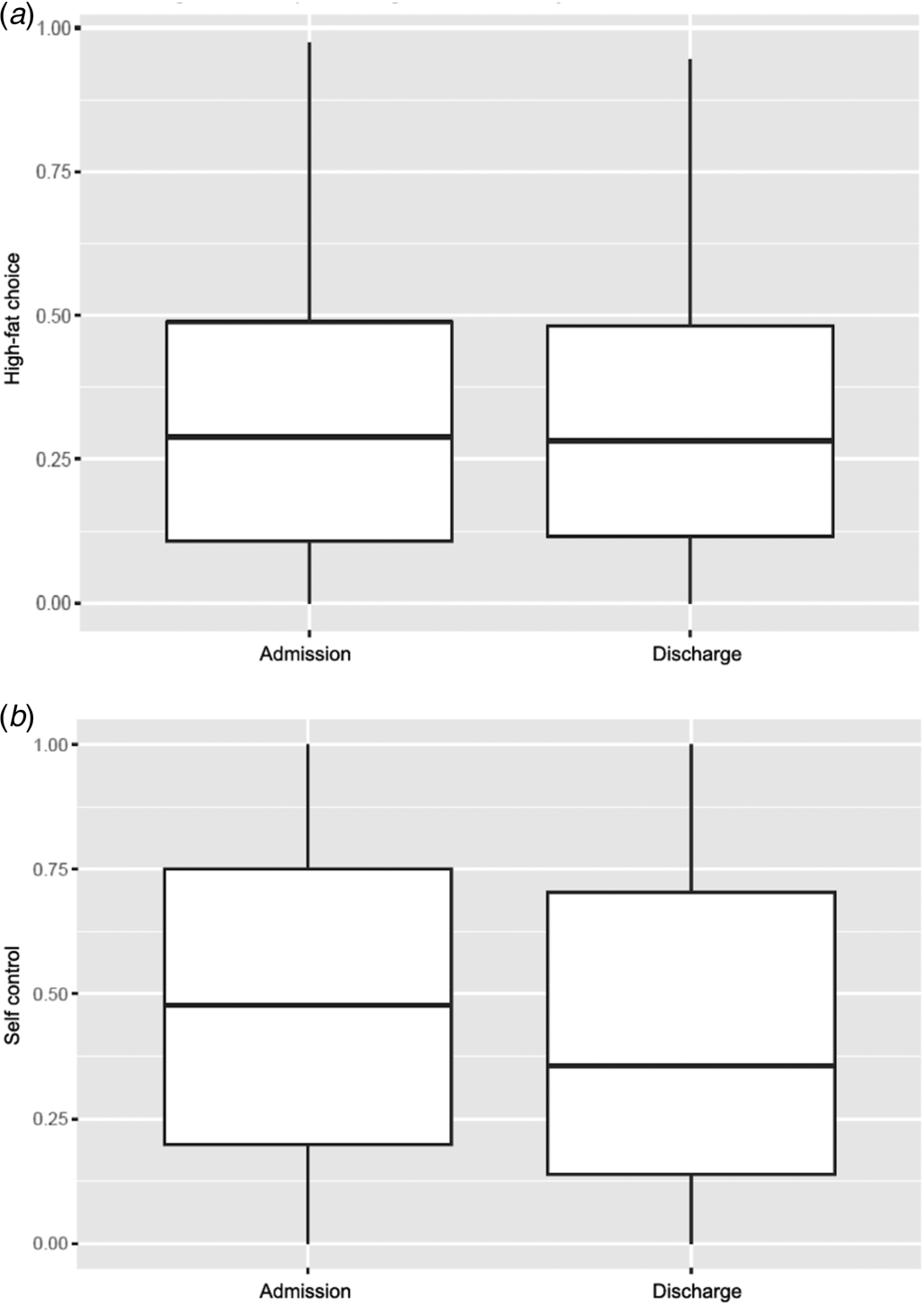
Figure 2. Food Choice Task performance at admission (time 1) and discharge (time 2). (a) Choices of high-fat items on the Food Choice Task did not change significantly during inpatient treatment. (b) The tendency to select items requiring self-control on trials where tastiness and healthiness conflicted did not change significantly during inpatient treatment.
Change in the proportion of trials in which high-fat foods were chosen over the reference food item was not significantly associated with BMI at discharge (r 86 = 0.13 [95% CI −0.08 to 0.33], p = 0.22) nor was change in the proportion of trials in which self-control was used (r 81 = 0.10 [95% CI −0.12 to 0.31], p = 0.39). Similarly, change in food choice variables was not significantly associated with BMI at discharge in linear regression analysis, adjusted for covariates (main effect of change in high-fat choices: β = 0.28 [95% CI −0.87 to 1.43], p = 0.64; main effect of change in use of self-control: β = 0.33 [95% CI −0.30 to 0.97], p = 0.31). Results did not change when sex assigned at birth was included as a covariate, and there was no significant effect of sex assigned at birth in the model.
Longer-term clinical status
Three participants were discharged within the prior year and therefore were not yet eligible for any follow-up assessments. Table 2 indicates categorical outcome by BMI of respondents and numbers of nonrespondents and participants not eligible due to insufficient time since discharge. Of the 85 eligible to participate in follow-up, 61 individuals (71.7%) participated in longitudinal assessments at least once and were included in analyses.
Table 2. Longitudinal follow-up interviews
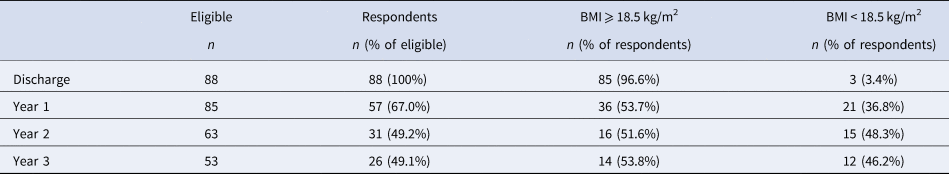
Notes: Eligible to participate in the follow-up interview is defined by time since discharge. That is, those who were discharged within a year of the study had not yet been approached for annual interviews.
BMI declined significantly over time (main effect of time: β = −0.89 [95% CI −1.15 to −0.62], p < 0.001). The change between admission and discharge in the proportion of high-fat foods selected was significantly associated with change in BMI over time after discharge (interaction: β = 1.08 [95% CI 0.19–1.97], p = 0.02) (see Fig. 3a) such that a greater increase in selection of high-fat food items was associated with less decline in weight during the 3 years of follow-up. In the longitudinal model, there was no significant association between change in high-fat food choice and BMI at discharge (i.e. time = 0), which is consistent with the short-term clinical status analysis, and there was no significant effect of subtype or age. There was a significant effect of duration of illness and BMI at admission such that a longer duration of illness and a lower BMI at admission were associated with a lower BMI during a 3-year follow-up (see online Supplementary Table S1).
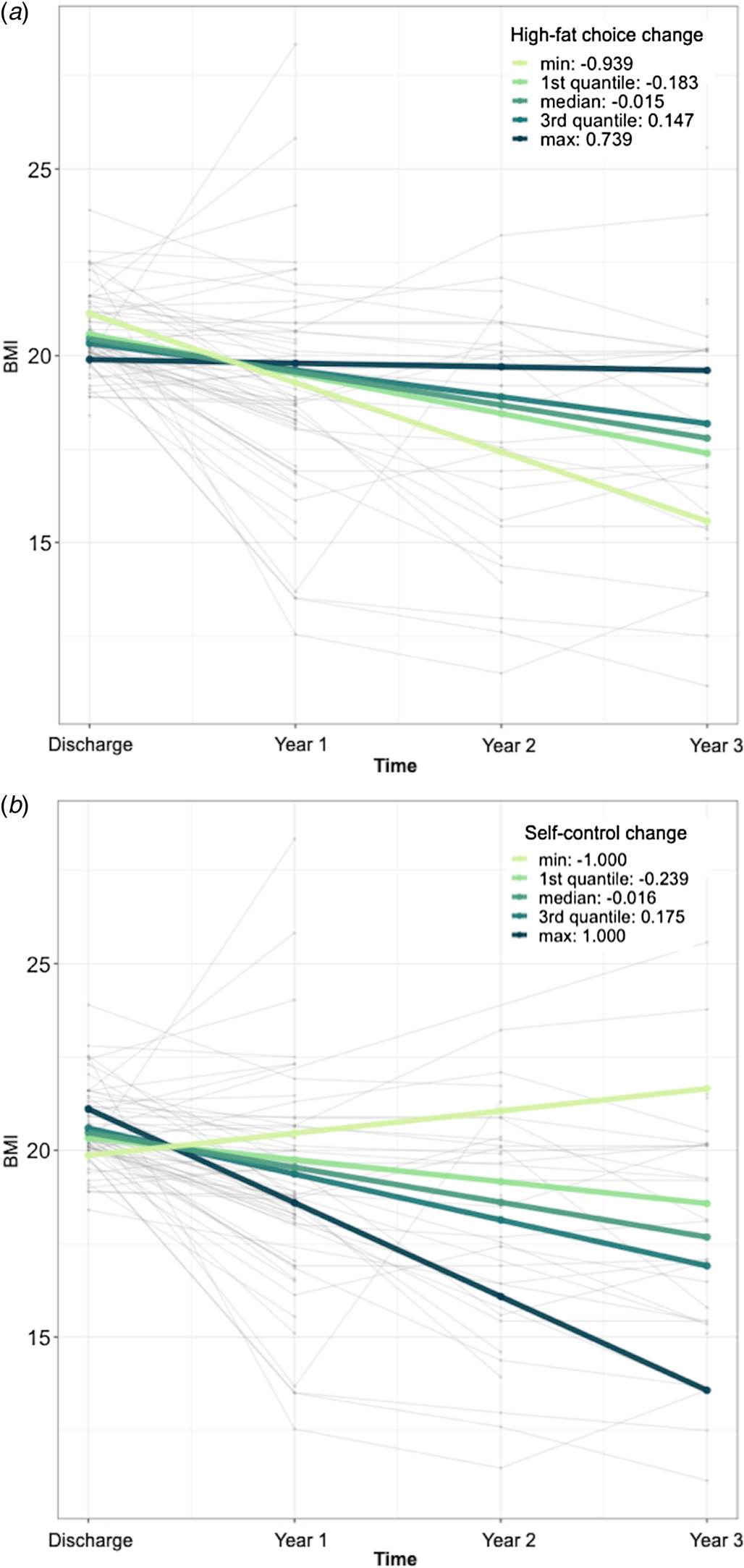
Figure 3. Long-term clinical status. BMI declined over time after hospital discharge; there was less decline in weight among individuals with AN who (a) increased high-fat choices and (b) decreased self-control choices over the course of inpatient treatment. Each fine line represents all available BMI values for one patient. Bolded lines represent the minimum, maximum, and quartile changes, as per the legend.
The change between admission and discharge in the use of self-control was also significantly associated with change in BMI over time (interaction: β = −1.57 [95% CI −2.54 to −0.62], p = 0.002) such that a greater decrease in the use of self-control was associated with less decline in weight (see Fig. 3b). As in the analysis of change in high-fat food choice, duration of illness and admission BMI were significantly associated with BMI over time (see online Supplementary Table S2). Including sex assigned at birth as a covariate did not change the results of the long-term analyses: high-fat interaction: β = =1.03, p = 0.03; self-control interaction: β = −1.53, p = 0.002.
The score on the EDE-Q did not change significantly over time (main effect: β = −0.13 [95% CI −0.27 to 0.01], p = 0.08). Change in food choice during inpatient treatment was not significantly associated with EDE-Q scores after discharge (interaction high-fat choice: β = −0.21 [95% CI −0.76 to 0.33], p = 0.45; interaction self-control: β = 0.22 [95% CI −0.29 to 0.73], p = 0.40; see online Supplementary Tables S3 and S4 for full results).
The proportion of patients who maintained a BMI ⩾ 18.5 kg/m2 did not change significantly over time (odds ratio of main effect: 0.13, p = 0.12). There was no statistically significant effect of change in high-fat choice (odds ratio 403.00, p = 0.17, CI 0.08–1.99 × 106) or in the use of self-control (odds Ratio 0.21, p = 0.35, CI 7.91 × 10−3, 5.62) during inpatient treatment on chance of maintaining a BMI ⩾ 18.5 kg/m2. There was no statistically significant effect of change in high-fat choice (odds ratio 1.76, p = 0.55) or in the use of self-control (odds ratio 0.72, p = 0.73) on the chance of maintaining a BMI ⩾ 18.5 kg/m2 and an EDE-Q < 2 at all available follow-up timepoints.
Discussion
In this study, 88 individuals with AN received inpatient treatment and completed a Food Choice Task (known to capture actual restrictive eating) at the time of hospital admission and at discharge. Inpatient treatment was associated with significant improvements in BMI and in the level of psychological symptoms characteristic of eating disorders, but, on average, there were no significant changes in the selection of high-fat foods or in self-control use when selecting food items for which there was a conflict between the tastiness and healthiness of the food item. An individual's changes in their selection of high-fat foods and in their use of self-control were not related to BMI at discharge. However, in a preliminary longitudinal analysis, changes in behavior on the Food Choice Task were related to longer-term course: increased selection of high-fat foods and decreased use of self-control between admission and discharge were associated with smaller decreases in weight over the following 3 years.
The current findings are consistent with a prior study, in which hospitalized patients with AN completed the Food Choice Task before and after full weight restoration during fMRI scanning. In that sample (which included a subset of the participants in the current study), there was also no significant change in the choice of high-fat foods, but the magnitude of change in behavior was significantly associated with the magnitude of change in associated neural activity in the anterior caudate (Foerde et al., Reference Foerde, Walsh, Dalack, Daw, Shohamy and Steinglass2021). Thus, some patients are able to make changes in their decisions of what foods to eat and these changes are associated with changes in neural circuits linked to food choice. The current study highlights that such changes are linked to longer-term outcome.
Furthermore, these findings underscore the continuing challenge of helping individuals with AN to change their eating behavior (Mayer et al., Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012) and the importance of food choice as a clinical target during acute treatment. Experienced inpatient treatment programs are largely successful in achieving normalization of weight (Murray et al., Reference Murray, Quintana, Loeb, Griffiths and Le Grange2019; Zeeck et al., Reference Zeeck, Herpertz-Dahlmann, Friederich, Brockmeyer, Resmark, Hagenah and Hartmann2018). Behavioral treatments use reinforcement of completion of meals and supervised meals to support eating, and the majority of patients are able to utilize this structure to overcome the restrictive drive that is part of the illness in order to achieve weight restoration. Improvement in weight is associated with numerous improvements in physical and mental health, including reductions in anxiety, depression, and obsessionality (Attia, Haiman, Walsh, & Flater, Reference Attia, Haiman, Walsh and Flater1998; Mayer et al., Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012; Sysko et al., Reference Sysko, Walsh, Schebendach and Wilson2005). Yet, the disturbance in eating behavior that is central to the disorder is extremely resistant to change: even after full weight restoration, treatment-seeking patients with AN limit caloric and fat intake when offered an ad libitum meal outside the structure of treatment (Mayer et al., Reference Mayer, Schebendach, Bodell, Shingleton and Walsh2012; Sysko et al., Reference Sysko, Walsh, Schebendach and Wilson2005). Additionally, one study found that improvement in eating self-efficacy (and not other aspects of self-efficacy) was associated with lower risk of weight loss at 6 months after discharge from an intensive treatment setting (Cooper, Guarda, Petterway, & Schreyer, Reference Cooper, Guarda, Petterway and Schreyer2021). These findings underscore the need to develop interventions that specifically aim to change an individual's food choices.
Attempts to identify key factors in illness trajectory have often yielded unmodifiable factors – including duration of illness and BMI at admission (Glasofer et al., Reference Glasofer, Muratore, Attia, Wu, Wang, Minkoff and Steinglass2020; Spitz, Aebi, Metzke, Walitza, & Steinhausen, Reference Spitz, Aebi, Metzke, Walitza and Steinhausen2022; Vall & Wade, Reference Vall and Wade2015), both of which were significantly associated with outcome in the current study. The current study identifies a modifiable treatment target: food choice. Family-based therapy (FBT) and cognitive behavioral therapy (CBT), both widely used in the treatment of AN, are interventions that include some features related to food choice. For example, in the final stage of FBT, the adolescent assumes responsibility for food decisions (Lock & Le Grange, Reference Lock and Le Grange2013); in CBT, the patient is encouraged to increase diet variety (Fairburn, Reference Fairburn2008). However, neither these nor other existing treatments of AN are centrally, primarily focused on food choice. Identifying novel treatments for AN is a priority, and recent efforts to improve long-term outcomes in AN have centered on the development of relapse prevention approaches. Food choice is an appealing treatment target because it can be modified even during acute treatment, with a potential impact on long-term outcome (i.e. relapse prevention). The Food Choice Task employed in the current study is freely accessible, easily administered, and can be used to assess the utility of treatment interventions.
Structured treatment programs include a range of settings (inpatient, residential, and partial hospital), all sharing a common behaviorally based approach to renourishment (Attia & Walsh, Reference Attia and Walsh2009). Such programs commonly incorporate a range of psychotherapeutic approaches adjunctive to the core behavioral approach. One approach focuses on adjunctive emotion-focused interventions (Thompson-Brenner et al., Reference Thompson-Brenner, Singh, Gardner, Brooks, Smith, Lowe and Boswell2021). Others have developed exposure-based interventions targeting eating-related anxiety (Cardi, Leppanen, Mataix-Cols, Campbell, & Treasure, Reference Cardi, Leppanen, Mataix-Cols, Campbell and Treasure2019; Steinglass et al., Reference Steinglass, Albano, Simpson, Wang, Zou, Attia and Walsh2014). Still others have used cognitive remediation as a way to target cognitive inflexibility during treatment (Hagan, Christensen, & Forbush, Reference Hagan, Christensen and Forbush2020). These approaches have yet to yield significant advances in long-term outcome and relapse rates remain high (Khalsa, Portnoff, McCurdy-McKinnon, & Feusner, Reference Khalsa, Portnoff, McCurdy-McKinnon and Feusner2017) likely because their impact on food choice is limited. Novel approaches that train flexibility in decision-making and enhance choices aligned with longer-term goals may more directly target change in eating choices. In anxiety disorders, attention-bias modification approaches have been shown to reduce anxiety symptoms and may be a particularly useful adjunctive treatment by directly targeting neurocognitive processes that perpetuate illness (Hang, Xu, Wang, Zhang, & Zhang, Reference Hang, Xu, Wang, Zhang and Zhang2021; Lazarov, Pine, & Bar-Haim, Reference Lazarov, Pine and Bar-Haim2017). Targeting decision-making about food may have even greater importance in AN, where current treatments are often disappointing (Brockmeyer, Friederich, & Schmidt, Reference Brockmeyer, Friederich and Schmidt2018). Neuromodulation has shown promise, in that a single-session administration of rTMS was associated with increased selection of high-fat food among inpatients with AN (Muratore et al., Reference Muratore, Bershad, Steinglass, Foerde, Gianini, Broft and Attia2021) and, among outpatients with AN, engagement of self-control has been shown to change with a course of treatment with repetitive transcranial magnetic stimulation (rTMS) (Dalton et al., Reference Dalton, Foerde, Bartholdy, McClelland, Kekic, Grycuk and Steinglass2020). Neurofeedback, an approach in its infancy, might be used to help an individual alter how they value calorie dense foods, in order to effect changes in choice of calorie dense foods (Sokunbi, Reference Sokunbi2018).
The current findings need to be considered in light of several limitations. The inpatient treatment program was highly successful in weight restoration such that there was limited variability in the discharge BMI. The restricted range of discharge BMIs may have reduced the opportunity to detect a relationship between changes in behavior on the Food Choice Task and short-term course (i.e. discharge BMI). Relatedly, those individuals who were not included in analyses due to completing only one timepoint (n = 19) might have differed in some respects from those who were included (although they did not differ in measured clinical and demographic factors, see online Supplementary Table S5); if we had been able to study a larger cohort who left without completing weight restoration, we would have been better equipped to test the relationship between short-term outcome (discharge BMI) and change in food choice. Participation in the annual follow-up assessments was low, reflecting a common challenge for the study of long-term outcomes. In a prior study, we have demonstrated that individuals who responded to the longitudinal surveys did not differ in age, sex, race, or severity of illness at the time of hospitalization from those who did not respond (they did differ in BMI at discharge, and were more likely to have binge-purge subtype of AN; Glasofer et al., 2020). A more robust research registry and more extensive efforts to remain in contact with participants might help improve participation over time, which would enhance the generalizability of findings. Follow-up weights were self-reported, but prior research has demonstrated that they are generally accurate (Glasofer et al., Reference Glasofer, Muratore, Attia, Wu, Wang, Minkoff and Steinglass2020). Furthermore, while race and ethnicity characteristics of this sample are consistent with population estimates (Udo & Grilo, Reference Udo and Grilo2018), treatment-seeking is less common in minoritized populations and this group may be under-represented in this study (Udo & Grilo, Reference Udo and Grilo2022). With the limited representation of minoritized populations in this study, it is not possible to evaluate if the findings would apply.
In summary, the current study demonstrates that changes in food choice during weight restoration treatment for AN using a computer task are associated with outcome over the following 3 years (Foerde et al., Reference Foerde, Steinglass, Shohamy and Walsh2015). Additional long-term follow-up is needed to clarify what changes in eating behavior are associated with sustained remission from AN. However, the association between increased high-fat choices, decreased use of self-control, and reduced weight loss over the following 3 years underscores the need for clinical treatment to focus on changing eating behavior.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291723002933
Acknowledgements
Supported by grants from COMPASS Pathways PLC, NIMH R01 MH105452, K24 MH113737.
Competing interests
Dr Steinglass reports receiving royalties and honoraria from UpToDate and Springer. Dr Walsh reports receiving royalties and honoraria from Guilford Publications, McGraw-Hill, Oxford University Press, UpToDate, and Wiley. Dr Attia reports receiving royalties from UpToDate and is Clinical Advisor to Equip Health. Dr Foerde, Dr Wang, Dr Lloyd, Ms. Touzeau, Ms. Ruggiero, and Ms. Feng report no financial relationships with commercial interests.








