Introduction
Suspiciousness, paranoid thinking, as well as feelings of alienation and isolation are key symptoms of psychotic disorders like schizophrenia. However, it is well-established that reports of psychotic-like experiences (PLE) are also frequently found in the general population, sparking a continuum model of psychosis-proneness (Claridge, Reference Claridge1997). In contrast to the schizophrenia prevalence of ~1% (Simeone, Ward, Rotella, Collins, & Windisch, Reference Simeone, Ward, Rotella, Collins and Windisch2015), psychotic phenomena (e.g. hallucinations) in the absence of the disorder have a lifetime prevalence of ~6–7% in the general population (Linscott & van Os, Reference Linscott and van Os2013; McGrath et al., Reference McGrath, Saha, Al-Hamzawi, Alonso, Bromet, Bruffaerts and Kessler2015). A considerable ~34% of healthy individuals between the age of 20 and 41 report at least mild psychotic signs (Rössler et al., Reference Rössler, Ajdacic-Gross, Haker, Rodgers, Müller and Hengartner2015), in child cohorts even up to >60% (Downs, Cullen, Barragan, & Laurens, Reference Downs, Cullen, Barragan and Laurens2013).
A continuous phenotypic marker of psychosis-proneness is schizotypy, a multidimensional set of schizophrenia-like personality traits that – similar to the manifest disorder – comprises positive (magical thinking, unusual perceptions and beliefs), negative (introversion, anhedonia), and disorganised (cognitive disorganisation, eccentricity) dimensions (Barrantes-Vidal, Grant, & Kwapil, Reference Barrantes-Vidal, Grant and Kwapil2015; Debbané & Barrantes-Vidal, Reference Debbané and Barrantes-Vidal2015; Grant, Reference Grant2015). Schizotypy can be conceptualised as a stable, underlying predisposition (Kwapil & Barrantes-Vidal, Reference Kwapil and Barrantes-Vidal2015; Kwapil, Chapman, & Chapman, Reference Kwapil, Chapman and Chapman1999) for more state-like phenomena like PLE, the (momentary) expression of psychotic experiences in non-clinical populations (Chapman & Chapman, Reference Chapman and Chapman1980; van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009), possibly extending towards ‘ultra-high risk’ states defined for early detection and prevention of transition into clinical states (Schultze-Lutter et al., Reference Schultze-Lutter, Michel, Schmidt, Schimmelmann, Maric and Salokangas2015).
As shown in Fig. 1, the concepts of schizotypy, PLE, and ultra-high risk (UHR) overlap substantially, yet contribute uniquely to conversion prediction, as shown for high schizotypy and UHR (Flückiger et al., Reference Flückiger, Ruhrmann, Debbané, Michel, Hubl, Schimmelmann and Schultze-Lutter2016; Michel et al., Reference Michel, Flückiger, Kindler, Hubl, Kaess and Schultze-Lutter2019). However, even in high schizotypy, conversion rates into manifest disorders are low – and importantly, rather than the symptom level itself, the level of distress associated with psychotic experiences has greater predictive value (Hanssen, Bak, Bijl, Vollebergh, & Os, Reference Hanssen, Bak, Bijl, Vollebergh and Os2005a; Hanssen, Krabbendam, De Graaf, Vollebergh, & Van Os, Reference Hanssen, Krabbendam, De Graaf, Vollebergh and Van Os2005b). At the same time, the association between PLEs and distress seems to be moderated by schizotypy: individuals showing high levels of schizotypy reported more PLEs, but at the same time less distress associated with them, compared to individuals with low trait schizotypy (Kline et al., Reference Kline, Wilson, Ereshefsky, Nugent, Pitts, Reeves and Schiffman2012).
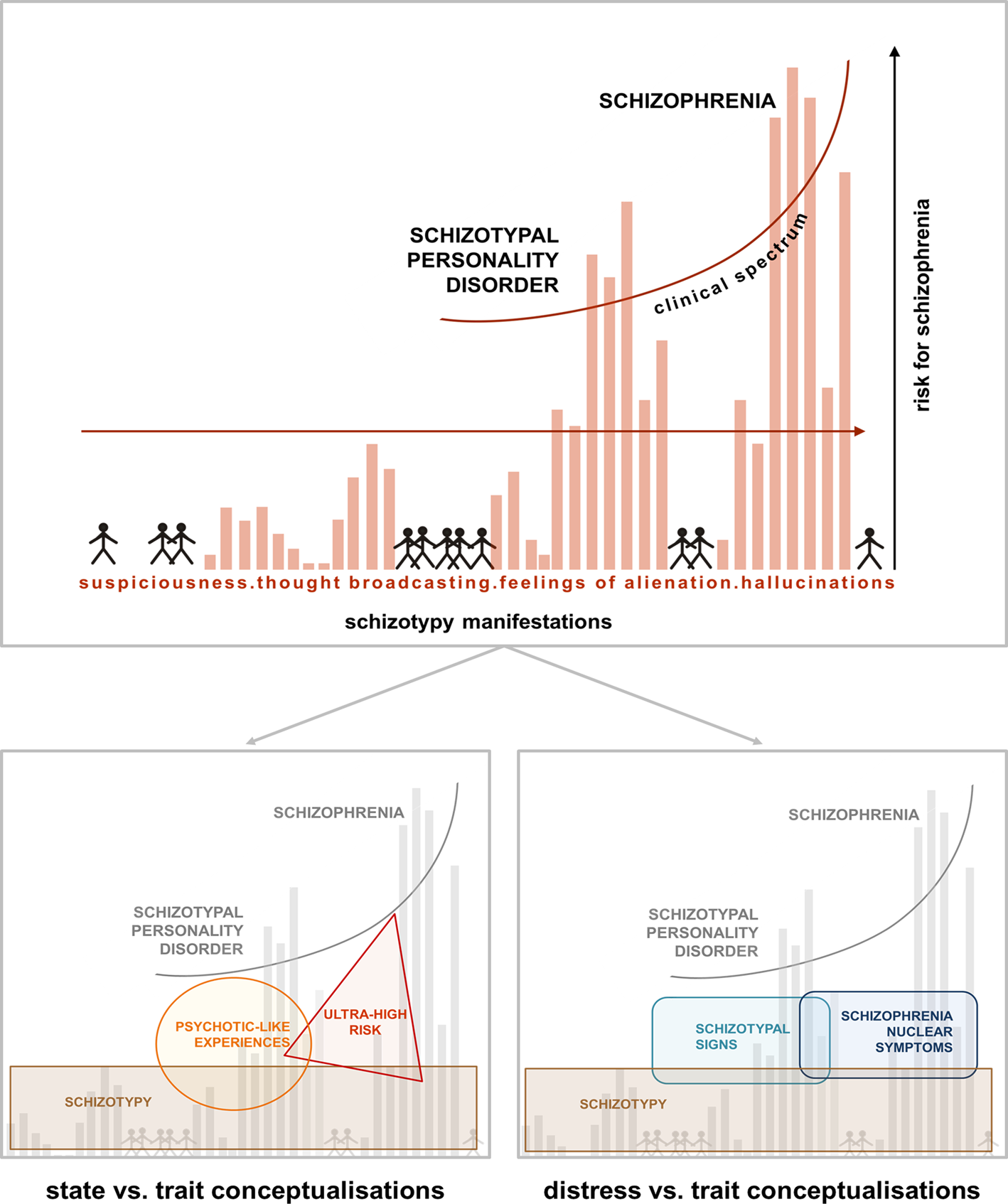
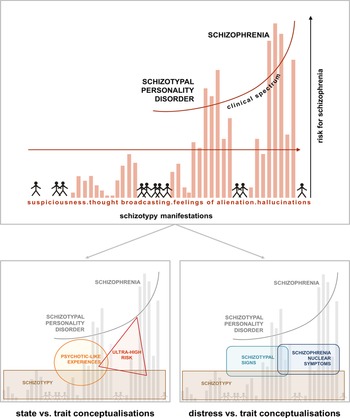
Fig. 1. Psychosis continuum model incorporating the STS v. SNS dimensions. Upper half [modified from Claridge and Beech (Reference Claridge, Beech, Raine, Lencz and Mednick1995)] shows a model of the psychosis continuum where, from the non-clinical towards the clinical parts of the spectrum, symptoms like suspiciousness, thought broadcasting, alienations, and hallucinations increase in intensity in the general population. The model emphasises a dimensional transition across this spectrum, where distress may play an important role in conversion probability. Lower half: within the non-clinical part of the spectrum, different concepts like schizotypy, PLEs and ultra-high risk have been used to capture either trait-like features or state-related clinical aspects (left). The model on the right, depicting STS and SNS, specifically focuses on the distress caused by more trait-related, distress-associated schizotypal personality features (STS) v. schizophrenia nuclear symptoms (SNS) closer to the clinical part of the spectrum (right). The overlap of STS and SNS acknowledges the dimensional nature of this alternative approach.
Complementing traditional measures of schizotypal traits, a recent line of research (Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007) has delineated two dimensions of distress-based assessment of state-like, subclinical psychotic experiences: schizotypal signs v. schizophrenia nuclear symptoms (Bakhshaie, Sharifi, & Amini, Reference Bakhshaie, Sharifi and Amini2011; Breetvelt et al., Reference Breetvelt, Boks, Numans, Selten, Sommer, Grobbee and Geerlings2010; Rössler, Hengartner, Ajdacic-Gross, Haker, & Angst, Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013, Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2014; Rössler et al., Reference Rössler, Hengartner, Ajdacic-Gross, Haker, Gamma and Angst2011a, Reference Rössler, Vetter, Müller, Gallo, Haker, Kawohl and Ajdacic-Gross2011b, Reference Rössler, Ajdacic-Gross, Haker, Rodgers, Müller and Hengartner2015; Zhornitsky, Tikàsz, Rizkallah, Chiasson, & Potvin, Reference Zhornitsky, Tikàsz, Rizkallah, Chiasson and Potvin2015). Assessed with the widely used symptom-checklist-90R (SCL-90R) symptom checklist (Derogatis, Reference Derogatis1977), a questionnaire capturing subjective distress symptoms across several psychological and physical dimensions, they show good internal consistency and validity, and small to medium-sized correlations with traditional schizotypy instruments such as the schizotypal personality questionnaire (Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007, Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013, Reference Rössler, Ajdacic-Gross, Haker, Rodgers, Müller and Hengartner2015). The item wording and response scale are presented in Table 1.
Table 1. Items of the SCL-90R scales schizotypal signs and schizophrenia nuclear symptoms with group means and standard deviations (s.d.)
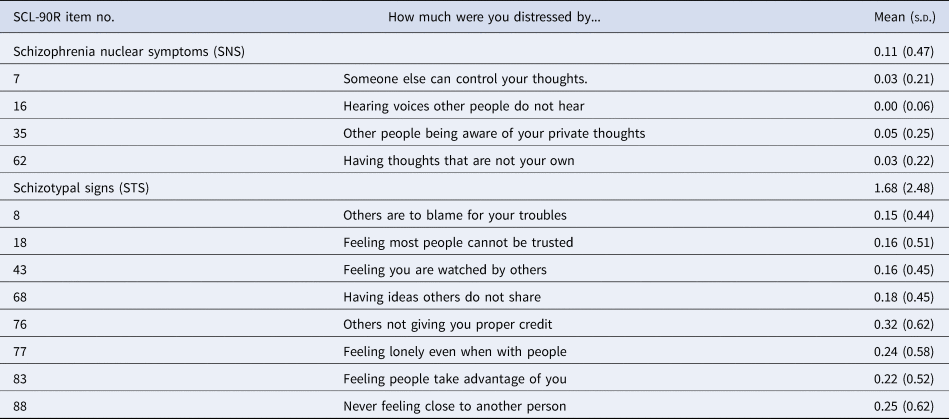
Items are rated (‘How much were you distressed by…’) over the last 4 weeks on a 5 point Likert scale between 0 (‘not at all’), 1 (‘a little bit’), 2 (‘moderately’), 3 (‘quite a bit’), and 4 (‘extremely’) for each item, resulting in scale ranges of 0–16 (SNS) and 0–32 (STS).
The schizotypal signs (STS) scale addresses distress evoked by interpersonal deficiencies, reduced capacity for close relationships, suspiciousness and paranoid ideation, resembling criteria for schizotypal personality disorder and positive and negative dimensions of schizotypy. Schizophrenia nuclear symptoms (SNS) assess distress caused by delusions of control, auditory hallucinations, thought-broadcasting and thought-intrusion, representing primarily positive symptoms of the schizophrenia spectrum.
The STS and SNS constructs represent an additional variation of subclinical markers with a special emphasis on distress (see Fig. 1). Although the two scales partially overlap with measures of schizotypy, rather than assessing a general personality disposition, they measure the level of distress caused by such experiences in a recent temporal interval. This level may vary across time, as suggested by studies investigating courses of PLE, STS, and SNS (Linscott & van Os, Reference Linscott and van Os2013; Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007). By focussing on symptom distress during the last 4 weeks, STS and SNS close a gap between extremely variable mood and stable personality structure (Franke, Reference Franke1995). While the temporally restricted assessment implies state-like rather than trait-like character of the scales, longitudinal studies suggest that the variability of state expression can be (partially) attributed to latent, stable traits and shows ‘the trait in action’, possibly representing responses to environmental challenges (Barrantes-Vidal et al., Reference Barrantes-Vidal, Grant and Kwapil2015; Rössler et al., Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013).
Growing evidence suggests that both state- (PLEs) and trait-like (schizotypy) expression of such attributes is associated with morphometric variation in certain brain regions. For schizotypy, this includes the precuneus as well as inferior and superior frontal and medial temporal cortical areas (Ettinger et al., Reference Ettinger, Williams, Meisenzahl, Möller, Kumari and Koutsouleris2012; Modinos et al., Reference Modinos, Mechelli, Ormel, Groenewold, Aleman and McGuire2010; Nenadic et al., Reference Nenadic, Lorenz, Langbein, Dietzek, Smesny, Schönfeld and Gaser2015b; Wang et al., Reference Wang, Yan, Yin, Fan, Cheung, Pantelis and Chan2015; Wiebels, Waldie, Roberts, & Park, Reference Wiebels, Waldie, Roberts and Park2016). Similarly, healthy individuals with PLEs as well as individuals at UHR for psychosis show variations in inferior temporal regions, insula and precuneus (Dietsche, Kircher, & Falkenberg, Reference Dietsche, Kircher and Falkenberg2017; Fusar-Poli et al., Reference Fusar-Poli, Borgwardt, Crescini, Deste, Kempton, Lawrie and Sacchetti2011; Nenadic et al., Reference Nenadic, Dietzek, Schönfeld, Lorenz, Gussew, Reichenbach and Smesny2015a; van Lutterveld, Diederen, Otte, & Sommer, Reference van Lutterveld, Diederen, Otte and Sommer2014a; van Lutterveld et al., Reference van Lutterveld, van den Heuvel, Diederen, de Weijer, Begemann, Brouwer and Sommer2014b). This suggests common neuroanatomical correlates along the psychosis spectrum (Modinos et al., Reference Modinos, Mechelli, Ormel, Groenewold, Aleman and McGuire2010), as those regions also overlap with alterations in schizophrenia (Dietsche et al., Reference Dietsche, Kircher and Falkenberg2017).
While previous literature strongly supports volumetric correlates, the association with brain surface morphometry, such as cortical folding or gyrification, still remains largely unknown. Opposed to the high plasticity of volumetric structure, cortical folding, determined during early brain development (Chi, Dooling, & Gilles, Reference Chi, Dooling and Gilles1977), is thought to be a sensitive and stable marker of neurodevelopmental variation (Nenadic, Yotter, Sauer, & Gaser, Reference Nenadic, Yotter, Sauer and Gaser2014; Yotter, Nenadic, Ziegler, Thompson, & Gaser, Reference Yotter, Nenadic, Ziegler, Thompson and Gaser2011). It might thus indicate neuronal processes long before symptom onset and serve as a surrogate of early neurodevelopmental insult. As altered gyrification patterns within superior temporal, prefrontal, and cingulate cortex have been identified both in psychosis and high risk, the parameter has been suggested as a neurodevelopmental marker for psychosis (Damme et al., Reference Damme, Gupta, Nusslock, Bernard, Orr and Mittal2019; Zuliani et al., Reference Zuliani, Delvecchio, Bonivento, Cattarinussi, Perlini, Bellani and Veneto Group2018).
STS and SNS are psychometrically well-validated, whereas their neuroanatomical correlates still remain unclear. Therefore, the aim of the present study was to test in a large, healthy cohort drawn from the general population the hypothesis that the phenomenologically delineated dimensions schizotypal signs and schizophrenia nuclear symptoms are associated with volume- and surface-based brain structural correlates similar to those found for schizophrenia and PLEs. Based on current evidence from brain imaging studies, we hypothesised reduced volume in frontal and medial temporal cortical areas and increased volume in the precuneus with increasing symptom load. In addition, we tested the hypothesis that altered gyrification in these regions, as seen in schizophrenia (Spalthoff, Gaser, & Nenadić, Reference Spalthoff, Gaser and Nenadić2018), would be related to schizophrenia nuclear symptoms.
Methods
Sample
We analysed data from 672 healthy participants [424 female (63.1%), 248 male (36.9%); mean age = 32.51 years, s.d. = 12.23], a subset of the FOR2107 cohort (Kircher et al., Reference Kircher, Wöhr, Nenadic, Schwarting, Schratt, Alferink and Dannlowski2018), a multi-centre study recruiting from the areas of Marburg and Münster in Germany. All experimental procedures were approved by the local ethics committees of the Medical Schools of the Universities of Marburg and Münster, respectively, in accordance with the current version of the Declaration of Helsinki. We included healthy adults between the age of 18 and 65 years. Exclusion criteria were current or former psychiatric disorders [assessed with SCID-I interviews (Wittchen, Wunderlich, Gruschwitz, & Zaudig, Reference Wittchen, Wunderlich, Gruschwitz and Zaudig1997) by trained raters], neurological, or other severe medical disorders, current drug use, verbal IQ<80 [estimated with Multiple Choice Word Test-B (Lehrl, Reference Lehrl1995)] as well as common magnetic resonance imaging (MRI) contraindications. All participants volunteered to take part in the study, gave written informed consent and received financial compensation afterwards.
Assessment of schizotypal signs and schizophrenia nuclear symptoms
All participants completed the German version (Franke, Reference Franke1995) of the SCL-90R-checklist (Derogatis, Reference Derogatis1977) as part of a larger test battery (Kircher et al., Reference Kircher, Wöhr, Nenadic, Schwarting, Schratt, Alferink and Dannlowski2018) within 14 days of MRI scanning. The SCL-90R is a well-established self-report questionnaire assessing the distress of 90 psychological symptoms across nine dimensions on a 5-point Likert scale, including the dimensions psychoticism and paranoid thinking. Participants were asked to rate symptoms over the past 4 weeks. Based on previous studies (Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007, Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013, Reference Rössler, Ajdacic-Gross, Haker, Rodgers, Müller and Hengartner2015), we computed the sum of the scales ‘schizotypal signs’ (STS; eight items) and ‘schizophrenia nuclear symptoms’ (SNS; four items) that were derived from factor analysis in a large longitudinal cohort study, based on the items of the original SCL-90R scales ‘paranoid ideation’ and ‘psychoticism’ (Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007). Table 1 shows a list of items for both scales and respective descriptive statistics. SCL-90R has been shown to possess good internal consistency and test-retest-reliability (Derogatis & Cleary, Reference Derogatis and Cleary1977; Schmitz, Hartkamp, & Franke, Reference Schmitz, Hartkamp and Franke2000). In the current sample, the scales show reliability (Cronbach's α) of α = 0.367 (SNS) and α = 0.729 (STS), comparable to data in previous publications: in a large longitudinal cohort study in a German-speaking Swiss sample, Rössler et al. (Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013) reported consistency values of α = 0.497 to 0.694 (mean 0.595) for SNS, and α = 0.800 to 0.869 (mean 0.821) for STS across the multiple time points. The STS and SNS subscales have been derived and validated in epidemiological studies (Rössler et al., Reference Rössler, Riecher-Rössler, Angst, Murray, Gamma, Eich and Gross2007, Reference Rössler, Ajdacic-Gross, Haker, Rodgers, Müller and Hengartner2015).
MRI acquisition
High resolution, T1-weighted structural images were acquired on a 3 T MRI system in Marburg (12-channel head matrix Rx-coil; Tim Trio, Siemens, Erlangen, Germany) or Münster (20-channel head matrix Rx-coil; Prisma, Siemens, Erlangen, Germany). At each site, a three-dimensional MPRAGE sequence with slice thickness = 1.0 mm, voxel size = 1.0 × 1.0 × 1.0 mm3, field of view FOV = 256 mm and the following parameters in Marburg: acquisition time of TA = 4:26 min, repetition time of TR = 1.9s, echo time TE = 2.26 ms, inversion time TI = 900 ms, 176 slices, flip angle = 7°; and Münster: TA = 4:58 min, TR = 2.13s, TE = 2.28 ms, TI = 900 ms, 192 slices, flip angle = 8°.
MRI data pre-processing
Pre-processing and voxel-based morphometry (VBM) analyses (Ashburner & Friston, Reference Ashburner and Friston2000) were executed using the pipeline of the CAT12 toolbox (version 1184, Structural Brain Mapping Group, Jena University Hospital, Jena, Germany) building on SPM12 (Statistical Parametric Mapping, Institute of Neurology, London, UK), running under MatLab (v2017a, The MathWorks, USA) with default parameter settings. Only subjects with at least satisfactory quality measures derived from CAT12 and complete questionnaire data were included in the analyses. Data from both centres were pooled based on extensive quality assurance protocols (Vogelbacher et al., Reference Vogelbacher, Möbius, Sommer, Schuster, Dannlowski, Kircher and Bopp2018).
For VBM analyses, images were segmented into grey matter, white matter, and cerebrospinal fluid and spatially normalised with the DARTEL algorithm (Ashburner, Reference Ashburner2007). All images passed visual quality control (inspection for artefacts and image quality) by experienced researchers (DG, UD) and the homogeneity control implemented in the CAT12 toolbox. Images were smoothed with a Gaussian kernel of 10 mm [full width at half maximum (FWHM)].
We extracted surfaced-based morphometry (SBM) parameters with the CAT12 toolbox, that uses a novel algorithm to extract the cortical surface (Dahnke, Yotter, & Gaser, Reference Dahnke, Yotter and Gaser2013), allowing to calculate additional information on cortical parameters. We analysed cortical gyrification, based on absolute mean curvature (Luders et al., Reference Luders, Thompson, Narr, Toga, Jancke and Gaser2006). Gyrification images were smoothed with a Gaussian kernel of 20 mm (FWHM).
Statistical analyses
Statistical analyses were conducted using general linear models in CAT12 with a multiple regression design. For both SCL-90R subscales (STS, SNS), separate models were set up to test for associations with grey matter volume (GMV) and gyrification, respectively. To control for the influence of confounding variables, age, sex and site were included in the model as nuisance variables. We also accounted for an Rx coil change after 386 of 445 scans at the Marburg site by including head coil as an additional nuisance variable, as suggested in extensive quality assurance studies (Vogelbacher et al., Reference Vogelbacher, Möbius, Sommer, Schuster, Dannlowski, Kircher and Bopp2018). In VBM analyses, total intracranial volume was included as an additional covariate. We analysed positive and negative correlations of SCL-90R subscale sum values (STS, SNS) with morphometric parameters in whole-brain analyses. Results were considered significant at p < 0.05 cluster-level Family Wise Error (FWE)-corrected for multiple comparisons after an initial cluster-forming threshold of p < 0.001.
Results
Demographic characteristics
Neither SNS nor STS was correlated with sex (r = 0.041, p = 0.289; r = −0.030, p = 0.433, respectively). STS was correlated with age (r = 0.077, p = 0.046), but SNS was not (r = 0.001, p = 0.971). SNS and STS showed a significant intercorrelation (r = 0.355, p = 1.9 × 10−21). There were significant differences in age (p = 7.3 × 10−11) and SNS (p = 0.023) between the Marburg and Münster sub-cohorts.
VBM results
Regression analyses revealed significant correlations between the two scales and clusters in the following brain regions (see Fig. 2):
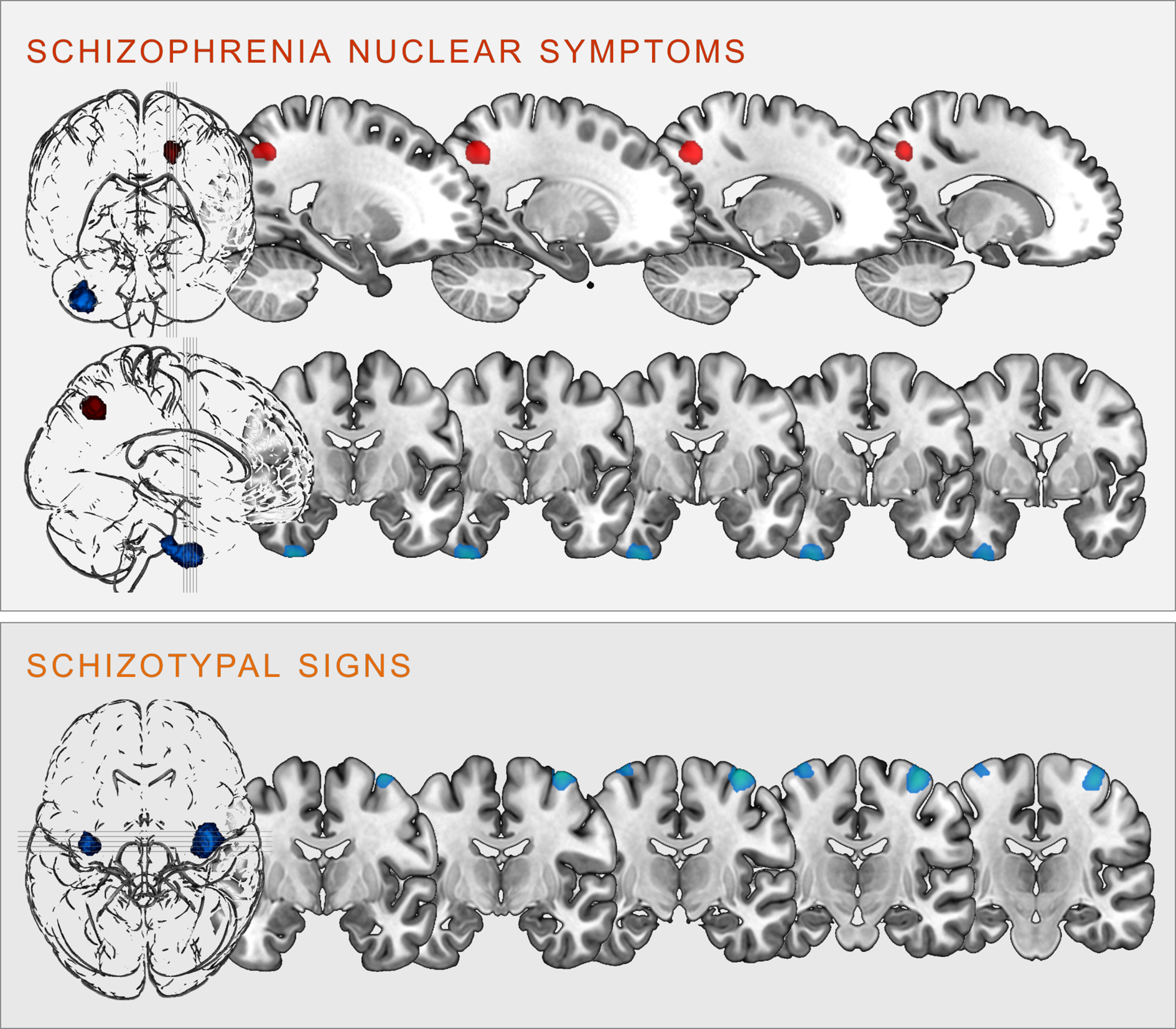
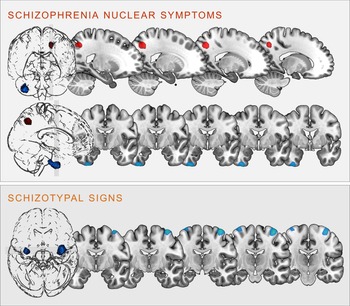
Fig. 2. Clusters of significant positive (red) and negative (blue) correlation between grey matter volume and SCL-90R-scales schizophrenia nuclear symptoms (upper panel) and schizotypal signs (lower panel) at p < 0.05, cluster-level FWE-corrected (illustration prepared with MRIcroGL; http://www.nitrc.org/projects/mricrogl).
Schizophrenia nuclear symptoms
SNS were positively correlated with GMV in the left superior parietal lobe, including parts of the left precuneus (k = 406 voxels, x/y/z = −18/−62/45, T = 4.56, p < 0.001 FWE cluster-level-corrected); and negatively correlated with GMV within the right inferior temporal gyrus (ITG), extending into the right fusiform gyrus (k = 915 voxels, x/y/z = 36/−8/-50, T = 3.92, p = 0.004 FWE cluster-level-corrected).
Schizotypal signs
STS were negatively correlated bilaterally with the GMV in the right (k = 1035, voxels, x/y/z = 38/−10/70, T = 4.16) and left (k = 298 voxels, x/y/z = −34/−10/75, T = 3.86) precentral gyrus (all p < 0.001 FWE cluster-level-corrected).
All of these clusters remained significant even after applying an additional Bonferroni correction by adjusting the significance threshold to p < 0.0125 (correcting for 4 tests).
SBM results
Schizophrenia nuclear symptoms
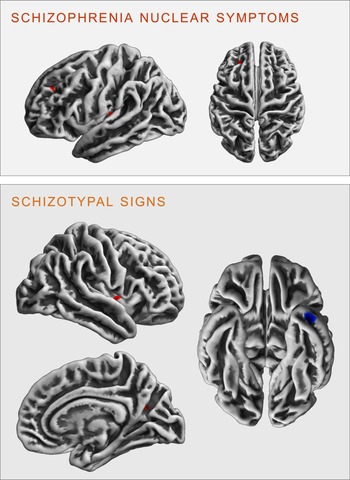
There were no significant FWE cluster level-corrected associations of the SNS score with gyrification. However, performing an exploratory analysis (p < 0.001 uncorrected), we identified a positive correlation of SNS with gyrification in the left insula (k = 27 voxels, x/y/z = −34/−24/5, T = 3.32) and the left rostral middle frontal gyrus (k = 19 voxels, x/y/z = −23/38/34, T = 3.30, see Fig. 3).
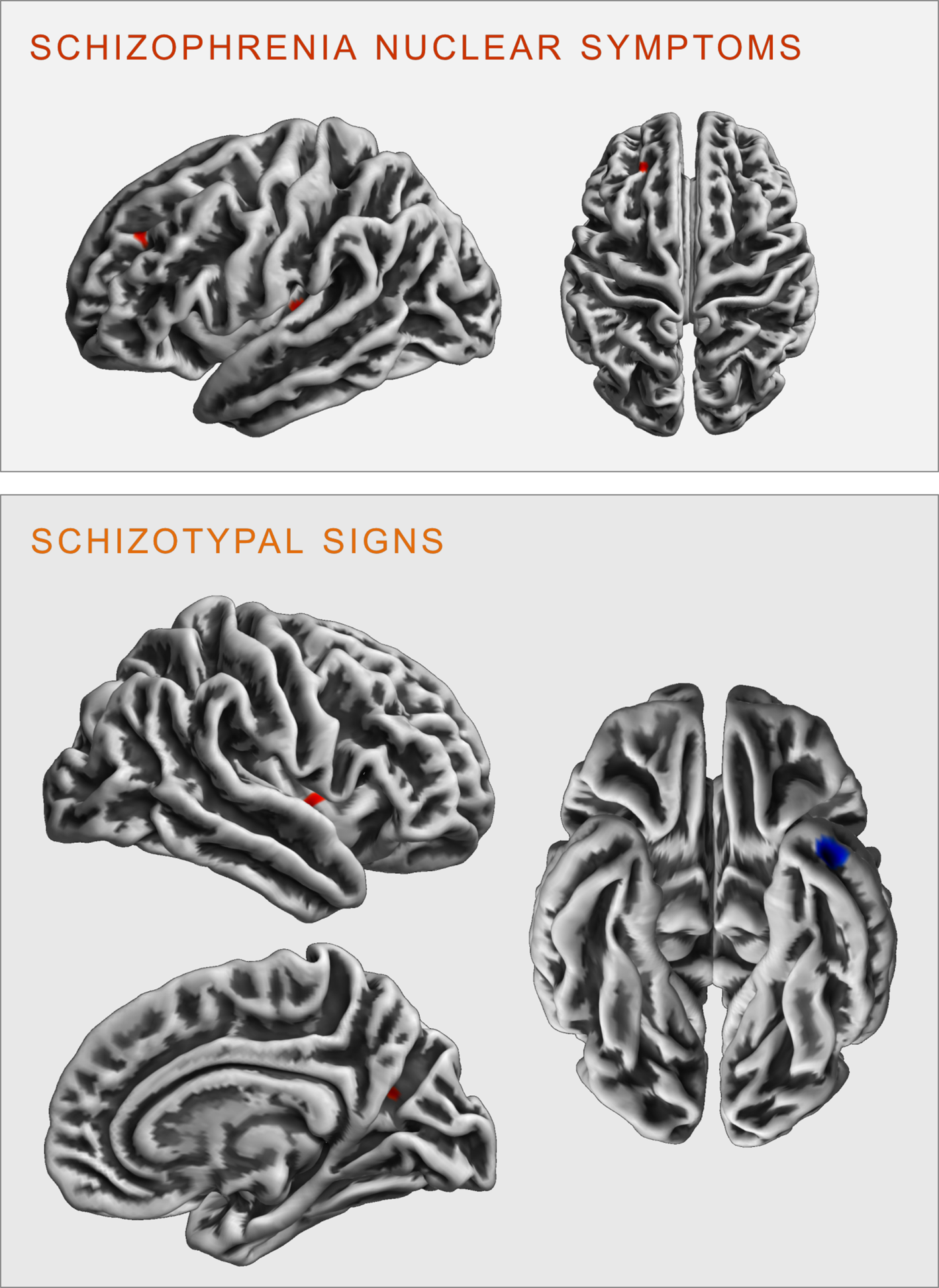
Fig. 3. Clusters of significant positive (red) and negative (blue) correlation between gyrification and SCL-90R-scales schizophrenia nuclear symptoms (upper panel) and schizotypal signs (lower panel) revealed in the exploratory analysis at p < 0.001 (uncorrected).
Schizotypal signs
There were no significant FWE cluster level-corrected associations of STS with gyrification. An exploratory analysis (p < 0.001 uncorrected), however, revealed that STS was positively correlated with gyrification in the right insula (k = 29 voxels, x/y/z = 40/−2/0, T = 3.31) and the right precuneus (k = 13 voxels, x/y/z = 23/−62/21, T = 3.27), as well as negatively correlated with gyrification in the left inferior/middle temporal gyrus (k = 42 voxels, x/y/z = −41/3/−37, T = 3.78 see Fig. 3).
Discussion
In the present study, we demonstrated that the level of distress related to PLEs in healthy adults is correlated with brain structural variation, similar to previously reported findings for measures of schizotypy and PLEs. Schizophrenia nuclear symptoms (SNS), capturing primarily positive, subpsychotic aspects closer to the clinical spectrum, were positively correlated with precuneus volume and negatively correlated with the volume in the ITG. Schizotypal signs (STS), reflecting a milder, personality trait-associated dimension of negative and positive symptoms, were negatively correlated with precentral volume. To date, this is the largest study of the association between brain structure and experiences from the schizotypal spectrum in healthy individuals, including a broader demographic spectrum than most preceding studies. Our results further endorse a dimensional model of neural correlates of schizotypy and psychosis-proneness and highlight the role of emotional appraisal of PLE in the healthy spectrum. This closes an existing gap between psychometrically assessed schizotypy (Claridge, Reference Claridge1997; Grant, Reference Grant2015) and clinically derived concepts (PLEs, UHR).
The main finding of our study is the disentangling of precuneus volume being linked to distress associated with positive, psychotic-like symptoms (SNS), but not to personality-related STS. This reinforces a spectrum model (shown in Fig. 1) in which sub-psychotic features closer to the clinical spectrum (as captured in SNS) are associated with precuneus variation. Therefore, our results provide evidence for differential brain structural associations of schizotypy and PLEs.
Our finding of the association with precuneus structure suggests its crucial role in mediating a higher risk stage. Precuneus structure has previously been linked to psychometrically assessed schizotypy, indeed this is one of the few findings that has been replicated across several studies (Modinos et al., Reference Modinos, Mechelli, Ormel, Groenewold, Aleman and McGuire2010, Reference Modinos, Egerton, McLaughlin, McMullen, Kumari, Lythgoe and Williams2018; Nenadic et al., Reference Nenadic, Lorenz, Langbein, Dietzek, Smesny, Schönfeld and Gaser2015b). There is also evidence of links to functional changes: non-clinical individuals with verbal hallucinations show increased precuneus activation (van Lutterveld et al., Reference van Lutterveld, van den Heuvel, Diederen, de Weijer, Begemann, Brouwer and Sommer2014b), and individuals at clinical high risk for psychosis show a failure to deactivate the precuneus in a working memory task (Falkenberg et al., Reference Falkenberg, Chaddock, Murray, McDonald, Modinos, Bramon and Allen2015). These structural and functional findings corroborate the role of the precuneus for symptoms within the psychosis spectrum.
The precuneus, part of the medial parietal cortex, has vast structural and functional connections with multiple brain regions and is thought to be involved in various higher-order cognitive processes (Cavanna & Trimble, Reference Cavanna and Trimble2006; Leech & Sharp, Reference Leech and Sharp2014; Zhang & Li, Reference Zhang and Li2012). Its involvement in self-reflection, discrimination of self v. others, and cognitive biases like thought-action fusion, reality distortion and self-referential ideas has been shown in studies in obsessive-compulsive disorder and the psychosis spectrum (Cavanna & Trimble, Reference Cavanna and Trimble2006; Jones & Bhattacharya, Reference Jones and Bhattacharya2014; Rikandi et al., Reference Rikandi, Pamilo, Mäntylä, Suvisaari, Kieseppä, Hari and Raij2017). Those findings are in line with our own results, linking precuneus volume to thought intrusion and broadcasting, verbal hallucinations and control delusions, as assessed by the SCL-90R schizophrenia nuclear symptoms scale.
Concerning our second finding, a negative correlation between SNS and inferior temporal GMV is in line with previously reported cortical thinning in the ITG linked to PLEs, i.e. verbal hallucinations in nonclinical individuals (van Lutterveld et al., Reference van Lutterveld, van den Heuvel, Diederen, de Weijer, Begemann, Brouwer and Sommer2014b). In one smaller study, volume reductions were also associated with attenuated psychotic symptoms in UHR individuals (Nenadic et al., Reference Nenadic, Dietzek, Schönfeld, Lorenz, Gussew, Reichenbach and Smesny2015a). ITG reductions were also part of a pattern distinguishing at-risk individuals with the later conversion from non-converters (Koutsouleris et al., Reference Koutsouleris, Gaser, Bottlender, Davatzikos, Decker, Jäger and Meisenzahl2010), and have been shown as longitudinal changes following the transition from risk status to psychosis onset (Borgwardt et al., Reference Borgwardt, McGuire, Aston, Gschwandtner, Pflüger, Stieglitz and Riecher-Rössler2008).
While these findings suggest PLEs and clinically derived markers to be associated with ITG structural variations, there is no evidence for such an association with schizotypy. This is consistent with the notion that SNS and STS might tap different parts of the psychosis spectrum. Given the overall low SNS scores in our sample, these findings appear all the more impressive, suggesting similar neuroanatomical correlates of even subtle subclinical variations as shown across the psychosis spectrum. It has to be considered, however, that given the high number of 0 scorers (as commonly seen in healthy population samples) leading to a skewed distribution of the scores, the use of a parametric design – although used in most other previous imaging studies of this kind – has considerable limitations. While large variation of morphometric parameters in subjects with 0 scores might obscure effects (increasing type II errors), we cannot exclude that additional associations might be identified in enriched samples recruiting a higher proportion of high SNS/STS subjects.
In our data, associations with the schizotypal signs scale were less prominent and restricted to a negative correlation in a cluster within the right and left precentral gyrus. While precentral gyrus volume decreases are generally associated with motor dysfunctions in schizophrenia (Tanskanen et al., Reference Tanskanen, Ridler, Murray, Haapea, Veijola, Jääskeläinen and Isohanni2010), and one study found a similar pattern in high risk and first episode individuals compared to healthy controls (Chang et al., Reference Chang, Womer, Bai, Zhou, Wei, Jiang and Wang2016), it does not generally feature in studies of PLEs in nonclinical individuals. There is, however, evidence for variations in adjacent regions, as reduced grey matter density in the dorsolateral prefrontal cortex has been reported in high v. low schizotypy (Wang et al., Reference Wang, Yan, Yin, Fan, Cheung, Pantelis and Chan2015). Postcentral grey matter reduction was reported in a clinical study with (female) patients with schizotypal personality disorder compared to controls (Koo et al., Reference Koo, Dickey, Park, Kubicki, Ji, Bouix and McCarley2006).
We did not detect associations of the STS scale with areas recently linked to psychometrically assessed schizotypy, such as the precuneus, prefrontal or temporal structures. STS in parts certainly overlaps with commonly used schizotypy measures, still, there are distinct differences: While other measures also often imply distress-proneness, the STS primarily and directly rates the distress level rather than that of the symptoms causing it. Also, the blending of positive (e.g. suspiciousness) and negative (e.g. social anhedonia) dimensions rather than distinguishing them may dilute effects. This should be considered as a limitation of the use of STS and SNS, especially with regard to comparability with studies using classical schizotypy measures. This is particularly the case for STS, because positive and negative symptoms as well as positive v. negative schizotypy measures might have different neurobiological correlates. Similar to previous studies, the SNS scale showed rather weak internal consistency in our sample, limiting the power to detect associations. While we still detected correlations with a region previously associated with positive symptoms, future studies might use other schizotypy measures with improved psychometric properties.
An important aspect of our finding is the direction of effects, i.e. the positive correlation of SNS and precuneus volume, and a negative correlation with ITG volume. Based on a simplified unidimensional linear association model (continuous volume decreases with increasing symptom load/disease), this might seem counterintuitive. However, comparing our findings to analyses of case-control studies in manifest schizophrenia, these overlap in location and direction of effects, showing both cortical thickening in the precuneus and thinning of ITG (van Erp et al., Reference van Erp, Walton, Hibar, Schmaal, Jiang and Glahn2018). Additionally, previous studies have suggested that the relation of subclinical and clinical markers might be modulated by additional variables like cognitive function (Meller, Ettinger, Grant, & Nenadić, Reference Meller, Ettinger, Grant and Nenadić2019), disorder duration (Faget-Agius et al., Reference Faget-Agius, Boyer, Padovani, Richieri, Mundler, Lançon and Guedj2012) or preserved insight (Egashira et al., Reference Egashira, Matsuo, Mihara, Nakano, Nakashima, Watanuki and Watanabe2014). Studies in other clinical dimensions, such as subclinical depressive and anxiety symptoms assessed with SCL90-R subscales (Besteher et al., Reference Besteher, Squarcina, Spalthoff, Bellani, Gaser, Brambilla and Nenadić2019), have suggested that nonlinear functions might describe the relationship. This hypothesis has not yet been tested in larger samples, which might include both major and minor disease variation (i.e. schizophrenia, schizotypal personality disorder, and other schizophrenia spectrum disorders).
Taken together, we show distinct associations with neuroanatomical correlates between SNS and STS, in line with recent findings in schizotypy. The spectrum of PLE towards psychotic symptoms is currently seen as a continuum (Claridge, Reference Claridge1997). This phenomenological continuum, however, is likely not monotonic and unidimensional but falls into several (potentially overlapping) dimensions or facets (Grant et al., Reference Grant, Green and Mason2018), represented by different brain structural (and functional) correlates and networks. By not fully distinguishing between experiences and associated distress, STS and SNS could be viewed as representative of an unidimensional, monotonic, rather than fully dimensional (Claridge, Reference Claridge1997) model, as the distinction between proneness for psychotic (-like) experiences and potential clinical relevance (i.a. schizophrenia-spectrum liability) is at the core of Claridge's conceptualisation of schizotypy (Grant et al., Reference Grant, Green and Mason2018; Schultze-Lutter, Nenadic, & Grant, Reference Schultze-Lutter, Nenadic and Grant2019).
It has been suggested that there are partially distinct susceptibilities to the schizophrenia spectrum (Barrantes-Vidal et al., Reference Barrantes-Vidal, Grant and Kwapil2015): While shared genetic variations render certain vulnerabilities to environmental events, other factors might buffer this influence and decrease the impact of schizophrenia risk factors by preserved or increased regional brain volume or function, or stabilised neurotransmitter activity (Siever & Davis, Reference Siever and Davis2004). These models have been mostly explored in schizophrenia v. schizotypal personality disorder patients, and only recently extended to the full spectrum including schizotypy in healthy individuals (Meller et al., Reference Meller, Ettinger, Grant and Nenadić2019). Our study further highlights the role of emotional appraisal that might modulate the impact of PLE on brain structure and subclinical v. clinical course.
While we did not find an association of the SNS and STS scales with gyrification patterns at corrected threshold levels, the exploratory, uncorrected analysis is of interest due to the associations with the precuneus as well as frontal and temporal regions. So far, there are no cortical folding or gyrification studies analysing associations of those dimensions with developmentally early and rather stable brain structural patterns, so additional studies are warranted. Although the SNS and STS are state-dependent, longitudinal analyses in a large, general population study showed that their variability is largely (75–89%) associated by stable traits (Rössler et al., Reference Rössler, Hengartner, Ajdacic-Gross, Haker and Angst2013). This, in line with our results, hints to a possible association of the scales with early, developmental markers, possibly indicating a vulnerability to the psychosis spectrum. In a developmental approach, it has also been argued that an underlying high level of psychometrically assessed schizotypy may constitute an increased liability to state-like subclinical PLE, suggesting an overlap of the partially distinct concepts (Debbané & Barrantes-Vidal, Reference Debbané and Barrantes-Vidal2015).
Our study addresses the nonclinical spectrum of schizotypal and sub-psychotic symptoms; therefore, as expected, the population shows low symptom loadings and restricted variance. This, however, only reduces statistical power, but should not invalidate the findings (Eysenck, Reference Eysenck1952) as it speaks to robustness of the effects.
In conclusion, our results support the notion that structural brain abnormalities in psychosis occur prior to or even independent of the development of full-blown symptoms, may progressively worsen over the course of the illness (Jung, Borgwardt, Fusar-Poli, & Kwon, Reference Jung, Borgwardt, Fusar-Poli and Kwon2012) and are modulated by emotional appraisal. As such, the phenomenological continuum seems to be reflected in an (at least partial) continuum of neurobiological correlates. It has to be pointed out, however, that the idea of a monotonic linear continuum appears to be overly simplified. Further, the existence of different concepts and phenomenological definitions as well as the use of instruments based thereon might contribute to mixed findings in current research, highlighting the importance of concise conceptualisation (Grant et al., Reference Grant, Green and Mason2018; Lee et al., Reference Lee, Chan, Chang, Lee, Hui and Chen2016; Oezgen & Grant, Reference Oezgen and Grant2018).
Acknowledgements
Acknowledgements and members by Work Package 1: Henrike Bröhl, Katharina Brosch, Bruno Dietsche, Rozbeh Elahi, Jennifer Engelen, Sabine Fischer, Jessica Heinen, Svenja Klingel, Felicitas Meier, Tina Meller, Torsten Sauder, Simon Schmitt, Frederike Stein, Annette Tittmar, Dilara Yüksel (Dept. of Psychiatry, Marburg University). Mechthild Wallnig, Rita Werner (Core-Facility Brainimaging, Marburg University). Carmen Schade-Brittinger, Maik Hahmann (Coordinating Centre for Clinical Trials, Marburg). Christian Bürger, Katharina Dohm, Fanni Dzvonyar, Verena Enneking, Stella Fingas, Katharina Förster, Janik Goltermann, Dominik Grotegerd, Hannah Lemke, Susanne Meinert, Nils Opel, Ronny Redlich, Jonathan Repple, Kordula Vorspohl, Bettina Walden, Dario Zaremba (Dept. of Psychiatry, University of Münster). Harald Kugel, Jochen Bauer, Walter Heindel, Birgit Vahrenkamp (Dept. of Clinical Radiology, University of Münster). WP6: Anastasia Benedyk, Miriam Bopp, Roman Keßler, Maximilian Lückel, Verena Schuster, Christoph Vogelbacher (Dept. of Psychiatry, Marburg University). Jens Sommer, Olaf Steinsträter (Core-Facility Brainimaging, Marburg University). Thomas W.D. Möbius (Institute of Medical Informatics and Statistics, Kiel University). All PIs take responsibility for the integrity of the respective study data and their components. All authors and co-authors had full access to all study data. The FOR2107 cohort project (WP1) was approved by the Ethics Committees of the Medical Faculties, University of Marburg (AZ:07/14) and University of Münster (AZ:2014-422-b-S). This work was partially supported through a FlexiFunds grant to IN and PG (FCMH grant number 2017_2_1_5) and the German Research Foundation (DFG) SFB-TRR58 (projects C09 and Z02 to UD), and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant number Dan3/012/17 to UD). This work is part of the German multicenter consortium ‘Neurobiology of Affective Disorders. A translational perspective on brain structure and function’, funded by the German Research Foundation (Deutsche Forschungs-gemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). Principal investigators (PIs) with respective areas of responsibility in the FOR2107 consortium are: Work Package WP1, FOR2107/MACS cohort and brainimaging: Tilo Kircher (speaker FOR2107; DFG grant numbers KI 588/14-1, KI 588/14-2), Udo Dannlowski (co-speaker FOR2107; DA 1151/5-1, DA 1151/5-2), Axel Krug (KR 3822/5-1, KR 3822/7-2), Igor Nenadic (NE 2254/1-2), Carsten Konrad (KO 4291/3-1). WP6, multi-method data analytics: Andreas Jansen (JA 1890/7-1, JA 1890/7-2), Tim Hahn (HA 7070/2-2), Bertram Müller-Myhsok (MU1315/8-2), Astrid Dempfle (DE 1614/3-1, DE 1614/3-2). CP2, administration. Tilo Kircher (KI 588/15-1, KI 588/17-1), Udo Dannlowski (DA 1151/6-1), Carsten Konrad (KO 4291/4-1). This work is part of the German multicenter consortium ‘Neurobiology of Affective Disorders. A translational perspective on brain structure and function’, funded by the German Research Foundation (Deutsche Forschungs-gemeinschaft DFG; Forschungsgruppe/Research Unit FOR2107). This work was partially supported through a FlexiFunds grant to IN and PG (FCMH grant number 2017_2_1_5) and the German Research Foundation (DFG) SFB-TRR58 (projects C09 and Z02 to UD), and the Interdisciplinary Center for Clinical Research (IZKF) of the medical faculty of Münster (grant number Dan3/012/17 to UD).
Conflicts of interest
Biomedical financial interests or potential conflicts of interest: Tilo Kircher received unrestricted educational grants from Servier, Janssen, Recordati, Aristo, Otsuka, neuraxpharm. All other authors declare no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.