The psychostimulants methylphenidate and dexamphetamine have an established place in the management of childhood attention-deficit hyperactivity disorder (ADHD) (MTA Cooperative Group, 1999). Immediate-release methylphenidate has a short plasma half-life and typically three doses per day are required. Traditional sustained-release preparations of methylphenidate may last up to 8 h but are often not as effective as multiple doses of immediate-release preparations (Reference Swanson, Gupta and GuintaSwanson et al, 1999) and have not been widely used in the UK. Concerta XL was the first once daily formulation of methylphenidate to be licensed in the UK, becoming available in February 2002. When ingested, a bolus of methylphenidate is delivered from its coating, followed by controlled delivery of the drug from the tablet’s core using Osmotic Release Oral System (OROS) technology. This sustained-release preparation is said to have a similar onset and duration of efficacy as three times daily dosing of immediate-release methylphenidate.
Method
This is an observational and retrospective study where three child psychiatrists and one community paediatrician working in Lincolnshire provided information on their patients who were either currently taking or had had a trial of the novel sustained-release psychostimulant at any time between February 2002 and February 2004. The patients were identified by the four treating doctors using a combination of computer case-load databases and personal case-load records. This method was the best available but cannot be guaranteed to have produced a comprehensive list. A standardised pro forma was used to collect information about the patients, their response to immediate-release psychostimulants prior to switching, the reasons for change and the outcomes of treatment with the new drug. Results were collated and analysed using the Statistical Package for the Social Sciences (SPSS) version 12 for Windows.
Results
Participants
A total of 103 young people were identified: 86 boys and 17 girls, with a mean age of 12 years 9 months (range 6–17 years). The mean time since diagnosis was 4 years (range 7–108 months). Neither the duration of treatment with immediate-release psychostimulants prior to switching nor the titration process informing the dose of immediate-release psychostimulant was recorded. Six young people had been treated with the sustained-release preparation as the first-line drug in the management of their ADHD: these children were excluded from the study (Fig. 1).
Reasons for switching
Clinicians were asked to indicate why they had changed their patients’ medication. More than one reason was given for each patient. The frequencies of different reasons given are shown in Fig. 2. The three most common reasons for switching were poor adherence to three times daily doses, erratic symptom control on immediate-release psychostimulants and a hope that patients would get a better therapeutic response.
Switching to an adequate dose
To check that clinicians had switched children from immediate-release psychostimulants to an adequate dose of the sustained-release preparation, we looked at all the children whose initial dose of immediate-release psychostimulant corresponded to the doses of methylphenidate given in the manufacturer’s dose algorithm (Janssen Cilag, 2002). There were 23 children initially taking the doses of immediate-release methylphenidate specified in the algorithm (methylphenidate 5 mg three times daily, 10 mg three times daily or 15 mg three times daily). Twenty of these children had been switched to the dose of the sustained-release preparation recommended by the manufacturer, one child had been switched to a higher dose and only two had been switched to a lower than recommended dose. In addition, we compared doses of the new drug of those who had a good response to the switching (mean 41.4 mg, s.d.=14.325) with those who had poor response (m 39.1 mg, s.d.=12.79). This difference was not statistically significant using either parametric (t=0.779, P=0.439) or non-parametric tests (Mann-Whitney test; P=0.554) (the samples were not normally distributed). We concluded that clinicians were generally using an adequate dose of the sustained-release preparation.
Outcomes after switching
A judgement of the effectiveness of a trial of the sustained-release preparation could be made for 92 young people. Clinicians rated the response to immediate-release psychostimulants as ‘minimally effective’, ‘ moderately effective’ or ‘very effective’. They were asked to rate the new treatment as ‘better’, ‘same’ or ‘ worse’. In all cases, the same clinician rated children on immediate-release psychostimulants and then when taking the new drug. Thirty-one children were rated as being ‘better’, 32 were rated as ‘ same’ and 29 were rated as ‘worse’.
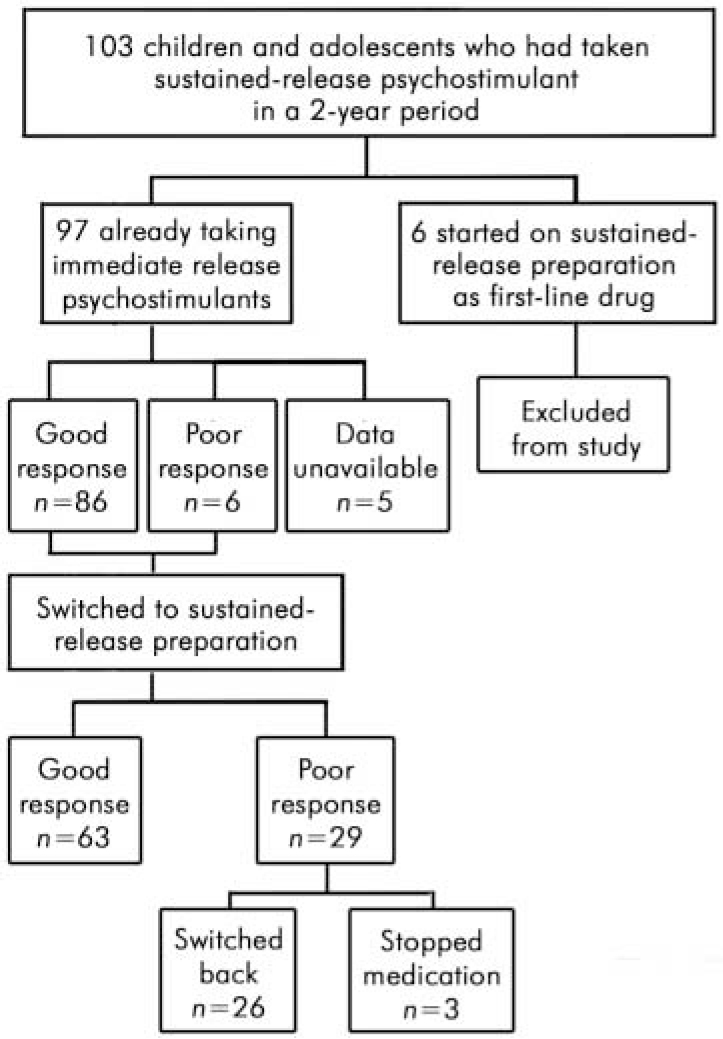
Fig. 1. Participants in the retrospective study of the new sustained-release psychostimulant (Concerta XL)
For the analysis the ‘better’ and ‘same’ categories were combined to make a single category ‘good’. Children rated as ‘ worse’ were said to have a ‘poor’ outcome. All but one of them (n=28) had previously had a ‘moderately effective’ or ‘ very effective’ response to immediate-release psychostimulants. The number of children with an adequate response to medication dropped from 86 on immediate-release psychostimulants to 63 on the new drug (Table 1). The outcome after switching was compared with the previous response using the McNemar test for paired nominal data. There was a significant decrease in good treatment response when children were switched (P<0.001) (Table 1 and Fig. 3).
Table 1. Treatment outcome on immediate release psychostimulants and after switching
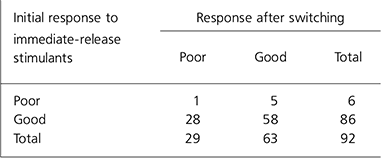
| Initial response to immediate-release stimulants | Response after switching | ||
|---|---|---|---|
| Poor | Good | Total | |
| Poor | 1 | 5 | 6 |
| Good | 28 | 58 | 86 |
| Total | 29 | 63 | 92 |
As the treatment outcome was a judgement made by the treating clinician without recourse to objective measurement, we analysed the outcome of switching in a second way. We assumed that if children and young people were switched back to immediate-release psychostimulants, then this indicated a clinically significant treatment failure. Twenty-six (27%) children and young people were switched back to immediate-release psychostimulants. The switch back occurred in the first few months of treatment (mean time of the switch back was 2.7 months, range 0.5–6 months). Manufacturer’s data suggest that the sustained-release preparation is identical to three times daily immediate-release methylphenidate and so one could assume that the expected failure rate would be zero. Based on this assumption the 27% rate of switching back is statistically significant (P<0.001) using binomial analysis. Even when we estimated that the expected treatment failure rate might be as high as 20% (allowing for patients’ dislike of a different preparation despite identical therapeutic equivalence) the statistical significance was maintained (P=0.038).
Influence of possible confounding variables
The characteristics of those children and young people who had a good response to the sustained-release preparation were compared with those who had a poor response. The tested variables were gender, age, time since diagnosis, erratic control on immediate-release psychostimulants, poor adherence to immediate-release psychostimulants and dose of new drug. Analysis using independent t-tests for continuous normally distributed variables, Mann-Whitney tests for continuous non-normally distributed variables and χ 2 tests for the other categorical variables failed to show any significant difference between poor responders and good responders (Table 2).
Table 2. Significance of possible confounding variables on the outcome of switching
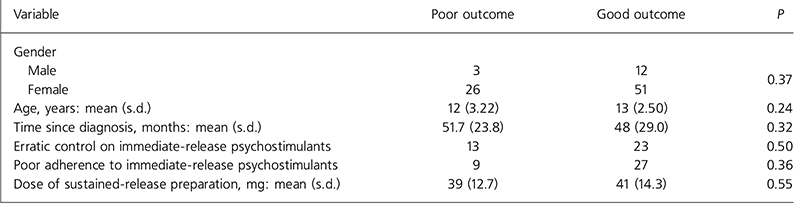
| Variable | Poor outcome | Good outcome | P |
|---|---|---|---|
| Gender | |||
| Male | 3 | 12 | 0.37 |
| Female | 26 | 51 | |
| Age, years: mean (s.d.) | 12 (3.22) | 13 (2.50) | 0.24 |
| Time since diagnosis, months: mean (s.d.) | 51.7 (23.8) | 48 (29.0) | 0.32 |
| Erratic control on immediate-release psychostimulants | 13 | 23 | 0.50 |
| Poor adherence to immediate-release psychostimulants | 9 | 27 | 0.36 |
| Dose of sustained-release preparation, mg: mean (s.d.) | 39 (12.7) | 41 (14.3) | 0.55 |
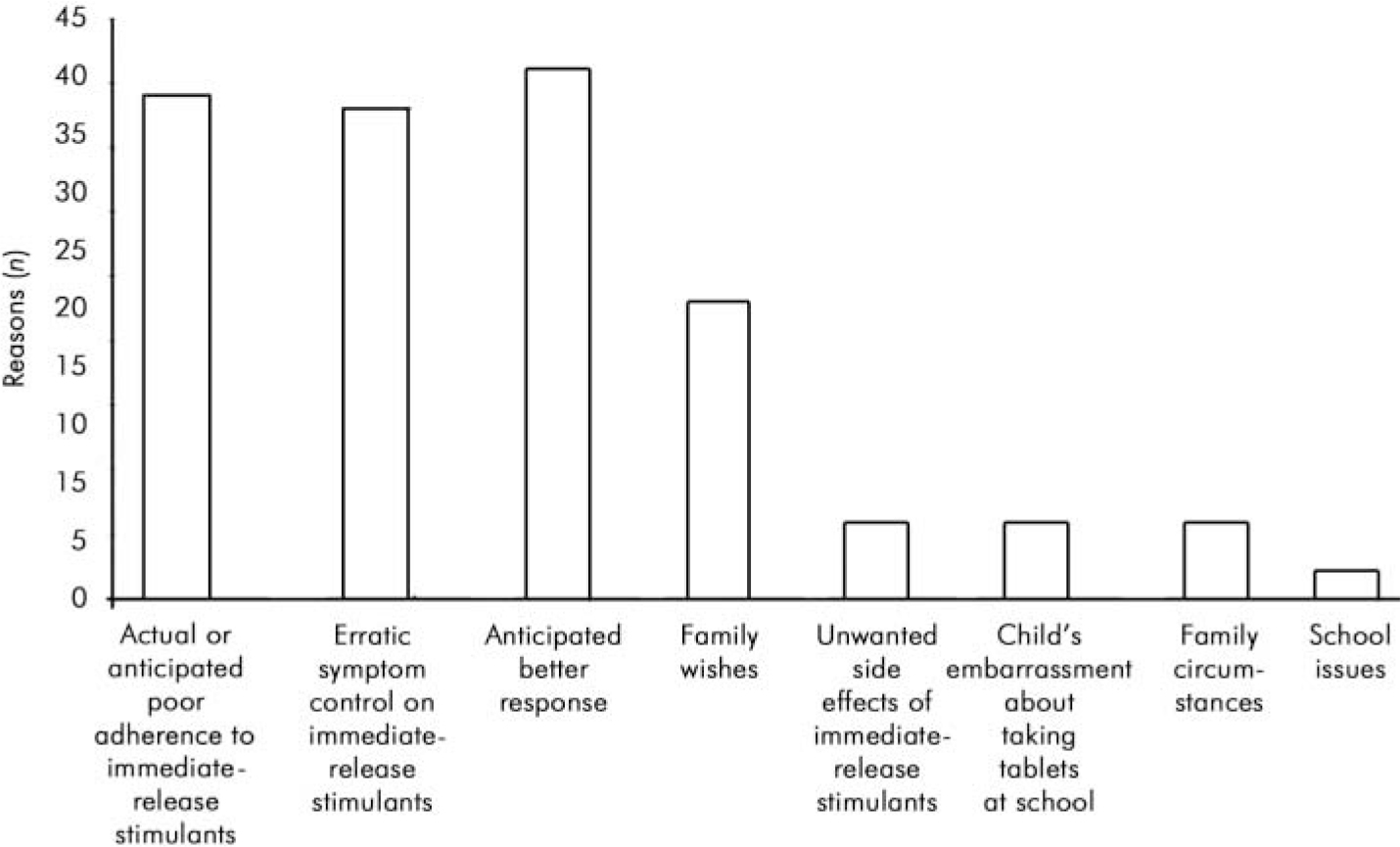
Fig. 2. Clinicians’ reasons for switching to sustained-release preparation.
Discussion
The new sustained-release psychostimulant (Concerta XL) has the same side-effect profile as immediate-release methylphenidate (Reference Pelham, Gnagy and Burrows-MacleanPelham et al, 2001). Its proposed advantage over immediate release methylphenidate is convenience and possible greater adherence to treatment (Reference RappleyRappley, 2001).
The results of this study of usual clinical care show that a significant proportion of young people (32%) switched from immediate-release psychostimulants to the sustained-release preparation experience a poor treatment response. This failure rate is higher than the generally accepted failure rate of immediate-release methylphenidate in the treatment of ADHD (20%). The discontinuation rate in this study is in fact lower than that reported by Hoare et al (Reference Hoare, Remschmidt and Medori2005) in their 12-month clinical trial (47%). Our results are a reminder that clinicians should be prescribing the new sustained-release preparation with the knowledge that not all young people will benefit and some would do better on immediate-release psychostimulants.
The failure rate demonstrated in this study may reflect the tendency to use inadequate doses for some young people. Although the clinicians switched individuals on lower doses of immediate-release methylphenidate to appropriate doses of the sustained-release preparation according to the manufacturer’s algorithm, anecdotal reports suggest some young people taking higher doses of immediate-release methylphenidate may need to be switched to 72 mg.
The results of the Multimodal Treatment Study of Children with Attention-Deficit Hyperactivity Disorder showed the benefits of careful monitoring of stimulant medication on patient outcome. In our study, the number of clinical contacts were not measured or controlled for. However, we assume that young people switched to a new drug are more likely to be monitored closely by clinicians than those on established treatment regimes. Hence we suggest that the beneficial effect of more intensive monitoring would have been evident once patients had been switched. This potentially positive influence on outcome was not evident.
Although anecdotal reports suggest that many specialists in the UK treating children who have ADHD are now prescribing the new preparation, we have been unable to find any systematic descriptions of this practice. Drug trials have shown a discontinuation rate of 17% in an 8-week randomised open-label trial of OROS methylphenidate v. usual clinical care with immediate-release methylphenidate (Reference Steele, Weiss and SwansonSteele et al, 2006) and a 15% discontinuation rate in a 3-week open label study (Reference Hoare, Remschmidt and MedoriHoare et al, 2005). When the study period of this second study was extended to 12 months, the discontinuation rate rose to 47%. Lack of efficacy was the main reason for discontinuation, with adverse events being the second most common reason. This clinical trial excluded some groups of children who are potentially hard to manage in clinical practice (e.g. children with psychiatric comorbidity and known non-responders to methylphenidate). In view of this bias, the fact that almost half of the sample discontinued treatment seems especially notable. However, the study was said to support the use of long-acting stimulants in preference to short-acting ones. The discontinuation rate of the new sustained-release preparation does not seem to be widely known.
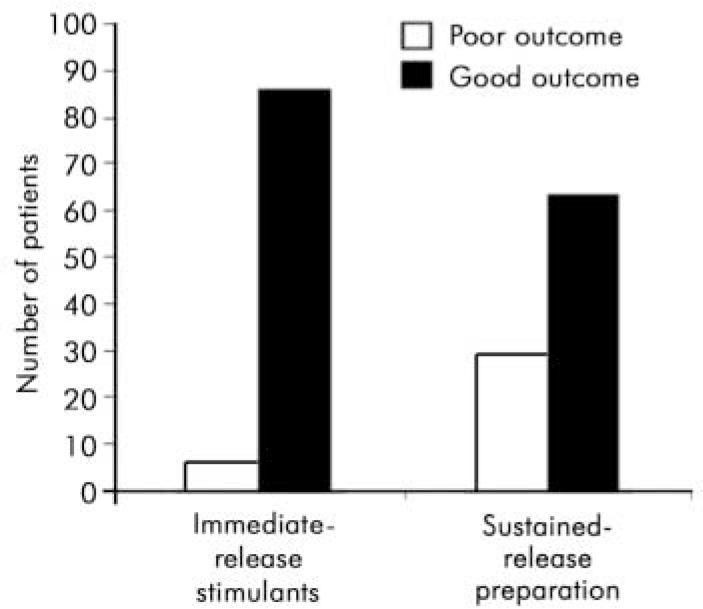
Fig. 3. Treatment outcome on immediate-release psychostimulants and after switching to Concerta XL.
Clinicians’ views of ‘benefit’ were not precisely defined in our study. The group of children said to have benefited from the switch probably experienced a mixture of improved symptom control, better adherence and patient preference. These results are based only on information from clinicians. Clearly, the views of the young people, their families and teachers would add to the validity of these findings and give a broader picture.
Another limitation of this study is that improvement was measured according to the clinician’s views and not an outcome scale. This was because of the study’s retrospective nature. We suggest that in day-to-day clinical practice, decisions about treatment are strongly influenced by clinicians’ views and so this measure of treatment success or failure has some face validity.
A prospective study including standardised outcome measurements and measures of patient adherence would add to our understanding of the place of the new sustained-release psychostimulant (Concerta XL) in the management of childhood ADHD.








eLetters
No eLetters have been published for this article.