Benzodiazepines are widely prescribed in psychiatric practice but because of their strong propensity to cause dependence, several guidelines have been published regulating their use (Table 1). There is concern that despite recommendations from national guidelines, benzodiazepines may be used for prolonged periods, thus risking dependence, and for inappropriate indications on acute psychiatric wards (Reference Vandel, Nezelof and BoninVandel et al, 1992; Reference Noble, Spiroulias and WhiteNoble et al, 1993).
Table 1. Current guidelines on the use of benzodiazepines

| Guideline | Recommendation |
|---|---|
| Committee on Safety of Medicines — anxiolytics (BMA & Royal Pharmaceutical Society, 2005) | Benzodiazepines are indicated for short-term relief (2-4 weeks of anxiety that is severe, disabling or subjecting the individual to unacceptable stress, occurring alone or in association with insomnia or short-term psychosomatic, organic or psychotic illness) |
| Committee on Safety of Medicines — hypnotics (BMA & Royal Pharmaceutical Society, 2005) | Benzodiazepines should be used when insomnia is severe, disabling or subjecting the individual to extreme distress |
| The Maudsley 2005-2006 Prescribing Guidelines (Reference Taylor, Paton and KerwinTaylor et al, 2005) | Benzodiazepines should not be prescribed as hypnotics or anxiolytics for longer than 4 weeks. |
| NICE Guideline for Anxiety (NICE, 2004) | Benzodiazepines should not be used in panic disorder and they should not be used beyond 2-4 weeks in generalised anxiety disorder |
| NICE Guideline for Schizophrenia (NICE, 2002) | Lorazepam can be used as part of rapid tranquillisation |
Lorazepam, a short-acting benzodiazepine, is commonly used to manage agitation associated with conditions such as psychosis, mania or hypomania (Reference Yildiz, Sachs and TurgayYildiz et al, 2003). Owing to the tendency for antipsychotic medication to cause extrapyramidal side-effects, lorazepam is an effective alternative in the treatment of acutely agitated patients (Reference Battaglia, Moss and RushBattaglia et al, 1997; Reference Foster, Kessel and BermanFoster et al, 1997; Reference Gillies, Beck and McCloudGillies et al, 2005). However, as short-acting benzodiazepines are associated with more severe withdrawal symptoms on cessation, the risk of inducing dependence is greater (British Medical Association & Royal Pharmaceutical Society, 2005).
This study examined the prescription and monitoring of lorazepam on three acute adult psychiatric wards at a university teaching hospital. The catchment areas of the four community mental health teams admitting patients to these wards were multicultural and urban.
Method
Retrospective data were collected from 102 consecutive in-patients on three acute adult psychiatric wards during April 2005. The information extracted by means of a standardised pro forma from prescription cards for each in-patient included:
-
• whether lorazepam was prescribed during the hospital admission
-
• whether the clinical indication for lorazepam prescription was documented by the prescribing doctor, either on the prescription card or in the medical notes
-
• whether the reason for administration of lorazepam on each occasion was documented in the patients’ notes by the nursing staff
-
• doses of lorazepam administered by nursing staff
-
• whether lorazepam prescription was reviewed and if so after what time period.
Results
There were 39 male (38.0%) and 63 female (62.0%) in-patients included in the study; the median age was 39 years (range 18–75 years). Diagnoses for in-patients were: 42 paranoid schizophrenia; 14 borderline personality disorder; 13 bipolar affective disorder; 8 alcohol-related disorders; 7 acute and transient psychotic disorder; 4 schizoaffective disorder; and 13 other diagnosis.
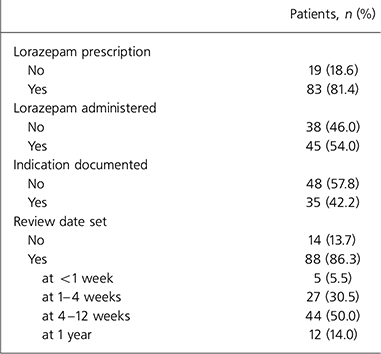
The extent of prescription and administration of lorazepam is shown in Tables 2 and 3. Of the total 102 patients, 83 (81.4%) were prescribed lorazepam during their admission. Of these, 38 (46.0%) were never administered the drug.
Table 2. Lorazepam prescription and monitoring
| Patients, n (%) | |
|---|---|
| Lorazepam prescription | |
| No | 19 (18.6) |
| Yes | 83 (81.4) |
| Lorazepam administered | |
| No | 38 (46.0) |
| Yes | 45 (54.0) |
| Indication documented | |
| No | 48 (57.8) |
| Yes | 35 (42.2) |
| Review date set | |
| No | 14 (13.7) |
| Yes | 88 (86.3) |
| at <1 week | 5 (5.5) |
| at 1-4 weeks | 27 (30.5) |
| at 4-12 weeks | 44 (50.0) |
| at 1 year | 12 (14.0) |
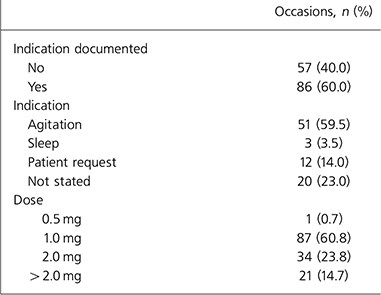
Table 3. Indications for lorazepam administration and dose given for 143 occasions
| Occasions, n (%) | |
|---|---|
| Indication documented | |
| No | 57 (40.0) |
| Yes | 86 (60.0) |
| Indication | |
| Agitation | 51 (59.5) |
| Sleep | 3 (3.5) |
| Patient request | 12 (14.0) |
| Not stated | 20 (23.0) |
| Dose | |
| 0.5 mg | 1 (0.7) |
| 1.0 mg | 87 (60.8) |
| 2.0 mg | 34 (23.8) |
| > 2.0 mg | 21 (14.7) |
The clinical indication for lorazepam prescription was documented by the doctor either in the medical notes or on the prescription card in 35 instances (42.2%). Lorazepam was administered on 143 occasions and indication for administration was documented on 86 (60.0%). Of these documented occasions, the indication was for agitation on 51 occasions (59.5%) and sleep on 3.5%. On 32 occasions (37%) the indication was unclear, in that it was administered at patient request on 12 occasions (14%) and not stated on 20 (23%).
More than 2 mg of lorazepam was administered on 21 occasions (14.7%) (1 in-patient was given 6 mg and 2 in-patients 5 mg each).
In 13.7% the lorazepam had not been reviewed and no review date had been set. In the other 86.3%, the majority had review dates set at more than 4 weeks (50% between 4 and 12 weeks and 14% at 1 year).
Discussion
Main findings
A large proportion of the patients prescribed lorazepam during their hospital admission were not actually administered the drug. This calls into question whether it was unnecessarily prescribed and whether closer consideration of its prescription is required at time of admission. There might be pressure for on-call junior doctors to prescribe lorazepam on admission in order to facilitate the management of patients on wards, especially out of hours. It also tends to be difficult to obtain information about patients at this time, and this may also lead to a lower threshold for prescription.
The clinical indication for lorazepam prescription was documented by the doctor for less than half of patients and this could lead to inappropriate administration. There was also relatively poor recording of both actual administration of lorazepam and the indication for which it was being given in the medical notes by nursing staff. Studies have previously reported a major reason for administering PRN medication was because patients had’requested’ it (Reference Gray, Smedley and ThomasGray et al, 1996). Similarly, the present study found that lorazepam was given at patient request on 14% of occasions.
The recommended dose of lorazepam for anxiety is 1–4 mg daily in divided doses (for older people this is half of the adult dose) and is 1–2 mg at bedtime for insomnia associated with anxiety. On 14.7% of occasions more than 2 mg was given on a single occasion. One patient was given 6 mg and two patients 5 mg each, despite documented maximum doses of 2 mg on the prescription charts. These doses were outside safe British National Formulary (BNF) limits (British Medical Association & Royal Pharmaceutical Society, 2005) and the reason for these excessive administrations appeared to be error rather than more extreme clinical presentations.
Regular review of continuing need for lorazepam is necessary owing to its propensity to cause dependence. In their case series of 227 in-patients, Vandel et al (Reference Vandel, Nezelof and Bonin1992) reported that hospitalisation was an inducer of benzodiazepine intake and dependence in 16% of the in-patients. Guidelines (Table 1) state that use of benzodiazepines should be limited to 2–4 weeks and therefore review of their use should be at less than 4 weeks. In 13.7% of patients no review date was set and of those cases that were reviewed or that had a review date set, only 36% were reviewed at less than 4 weeks. In 14% of patients the review date was set at 1 year, by which stage dependence would be highly likely. However, this study does not differentiate intermittent, which is less likely to induce dependence, from regular lorazepam use.
Implications
This study has identified scope for improvement both in the prescription and monitoring of lorazepam. It has highlighted possible excess and automatic prescription of lorazepam, inadequate documentation by both prescribers and administrators with regard to indication for use, as well as a lack of appropriate review of prescriptions on the three psychiatric wards considered. These practices risk inappropriate use and dependence if lorazepam is administered on a regular basis.
Clearer local guidelines should be in place to regulate the use of lorazepam. The reason why the patient was administered lorazepam by the nursing staff should be documented on each occasion in order to evaluate the continued need for lorazepam, the appropriateness of prescription as well as to monitor the mental state of the patient. Mandatory documentation of indication for lorazepam use on the prescription card by prescribing doctors should reduce its administration by nursing staff for inappropriate indications, for example at patient request. It would be helpful to liaise with hospital pharmacy departments regarding a possible change in configuration of prescription cards, in particular the creation of a space on the prescription card for the indication to be entered might lead to improved documentation. Review dates of less than 4 weeks should be set at the time of prescription to reduce unnecessarily prolonged prescriptions. In light of guidelines advising against long-term use, record should be kept of patients who are administered lorazepam regularly for more than a 2-week period to identify those at risk of developing dependence. Finally, similar investigation of other drugs likely to cause dependence, such as zopiclone and other benzodiazepines, would also be useful.
Declaration of interest
None.






eLetters
No eLetters have been published for this article.