Poor rates of compliance are a problem for the treatment of schizophrenia and have been estimated to be between 11% and 80% (Reference KaneKane, 1989). One of the many possible factors that can influence compliance is adverse medication effects and reduction of these may have a favourable effect on compliance rates (Reference BarnesBarnes, 1989).
Since the reintroduction of clozapine (Reference Kane, Honingfield and SingerKane et al, 1988), a number of antipsychotic drugs have been produced that attempt to mimic its pharmacological profile - the so-called ‘atypical’ antipsychotic agents. One such compound is olanzapine. This drug has been shown in several large trials to be as efficacious at controlling the symptoms of schizophrenia as haloperidol, but not to differ from placebo in incidence of extrapyramidal side-effects (EPS) (Beasley et al, Reference Beasley, Sanger and Satterlee1996a , Reference Beasley, Tollefson and Tran b , Reference Beasley, Hamilton and Crawford1997; Reference Tollefson, Beasley and TranTollefson et al, 1997). Whether the greater tolerability of such atypical drugs translates into better compliance has yet to be shown.
This study aimed to compare the attitude to medication of two groups of patients with schizophrenia, one taking olanzapine and another taking traditional antipsychotic medications. The hypothesis was that the greater tolerability of olanzapine owing to a lower incidence of EPS would lead to an improved attitude to medication in the olanzapine group. In addition, subjective quality of life in the two groups was assessed anticipating that decreased EPS in the olanzapine group would also result in better quality of life.
Method
All patients were recruited from acute adult services at South Kensington and Chelsea Mental Health Centre, London. Two groups of patients with a clinical diagnosis of schizophrenia were studied. These were 20 patients (five in-patients, 15 out-patients) receiving between 10 mg and 20 mg of olanzapine and 20 patients receiving depot traditional antipsychotic medication. All patients had been receiving their respective treatment for at least 6 weeks prior to entering the study. Patients were excluded if they were receiving any other psychotropic medication or if they were considered, on clinical grounds, to have been non-compliant in the 6 weeks prior to interview. Anticholinergic medication was not an exclusion factor.
For each subject, the following data were collected: age, gender, length of illness in years and length of time on medication in weeks. The following rating scales for assessing symptoms and side-effects were used: Clinical Global Impressions - Severity of Illness Scale (Reference GuyGuy, 1976), the Brief Psychiatric Rating Scale (BPRS; Reference Overall and GorhamOverall & Gorham, 1962), the Simpson-Angus Scale for EPS (Reference Simpson and AngusSimpson & Angus, 1970) and the Barnes Akathisia Rating Scale (BARS; Reference BarnesBarnes, 1989). In addition, the Rating of Medication Influences in Schizophrenia (ROMI; Reference Weiden, Rapkin and MattWeiden et al, 1994) and the Lancashire Quality of Life Profile (LQOLP, Reference OliverOliver, 1991) were administered. The ROMI is a scale designed to explore attitudes to medication, which underpin compliance and non-compliance. The LQOLP is a scale that measures quality of life in each of nine domains and also contains three general sub-scales for global well-being, affect balance and self-concept.
Groups were compared using non-parametric statistical tests (SPSS statistical package). Data on a nominal scale were compared using the Chi-square (χ2) test. Data on ordinal or interval scales were analysed using the Mann-Whitney U Test. Correlations were calculated using Spearman's test.
Findings
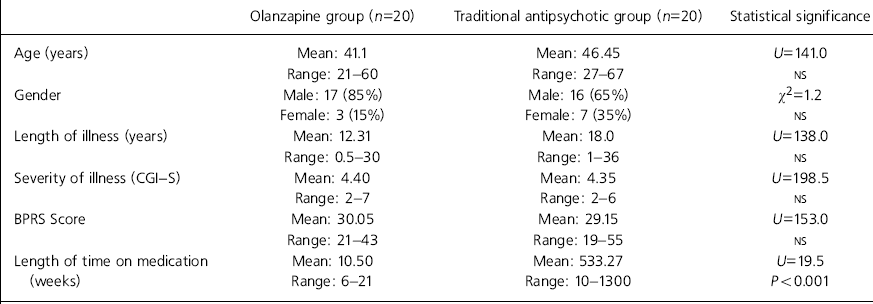
As can be seen in Table 1, the two groups were well matched for age, gender, length of illness, severity of illness and psychopathology (using the BPRS). Accurate data on length of treatment were available for all patients in the olanzapine group and for 11 in the traditional antipsychotic group; this was significantly longer in the latter group.
Table 1. Group characteristics
| Olanzapine group (n=20) | Traditional antipsychotic group (n=20) | Statistical significance | |
|---|---|---|---|
| Age (years) | Mean: 41.1 | Mean: 46.45 | U=141.0 |
| Range: 21-60 | Range: 27-67 | NS | |
| Gender | Male: 17 (85%) | Male: 16 (65%) | χ2=1.2 |
| Female: 3 (15%) | Female: 7 (35%) | NS | |
| Length of illness (years) | Mean: 12.31 | Mean: 18.0 | U=138.0 |
| Range: 0.5-30 | Range: 1-36 | NS | |
| Severity of illness (CGI-S) | Mean: 4.40 | Mean: 4.35 | U=198.5 |
| Range: 2-7 | Range: 2-6 | NS | |
| BPRS Score | Mean: 30.05 | Mean: 29.15 | U=153.0 |
| Range: 21-43 | Range: 19-55 | NS | |
| Length of time on medication | Mean: 10.50 | Mean: 533.27 | U=19.5 |
| (weeks) | Range: 6-21 | Range: 10-1300 | P<0.001 |
Table 2 shows that significantly more patients receiving traditional antipsychotic medication demonstrated EPS than those receiving olanzapine. In addition, akathisia was more prominent in the traditional antipsychotic group than in the olanzapine group (using the BARS).
Table 2. Medication side-effects
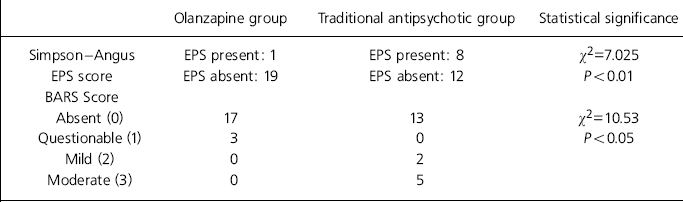
| Olanzapine group | Traditional antipsychotic group | Statistical significance | |
|---|---|---|---|
| Simpson—Angus | EPS present: 1 | EPS present: 8 | χ2=7.025 |
| EPS score | EPS absent: 19 | EPS absent: 12 | P<0.01 |
| BARS Score | |||
| Absent (0) | 17 | 13 | χ2=10.53 |
| Questionable (1) | 3 | 0 | P<0.05 |
| Mild (2) | 0 | 2 | |
| Moderate (3) | 0 | 5 |
There were no significant differences between the two groups with respect to the global quality of life scores: affect balance, self concept and global well-being; or for the nine more specific domains (range of Mann-Whitney Us=137.5-198.5, NS). To examine whether quality of life might be related to EPS in the traditional antipsychotic group, the global quality of life scores were compared for those with (n=8) and without (n=12) EPS. While there was no significant difference between the groups for self concept (Mann-Whitney U=35, NS) or global well-being (Mann-Whitney U=33.5, NS), the EPS group scored significantly lower on the affect balance scale (Mann-Whitney U=21, P<0.05).
On the ROMI, patients were shown seven statements that might reflect reasons for compliance and 13 for non-compliance, and were required to indicate the level of agreement between each statement and their own attitude to medication with the aid of a three-point scale: strong, mild, none. More patients in the olanzapine group agreed with the statement that they were taking their medication because they felt it stopped their symptoms returning (85% v. 55%, χ2=3.903, P<0.05). There were no differences for the remaining statements (range of χ2=0.04-1.71, NS). Following Weiden (Reference Weiden, Tamminga and Schultz1991), the seven compliance factors were collapsed into broader reasons: medication affinity, influence of others and prevention. Table 3 shows that there was no difference between the two groups for the former two factors. However, significantly more patients receiving olanzapine scored full marks for the ‘prevention’ factor. There was no significant difference between the groups for any of the non-compliance factors. However, there was a trend for more patients on traditional depot antipsychotic medication to cite “embarrassment about taking medication” as a reason for potential non-compliance (90% v. 65%, χ2=3.584, P=0.058).
Table 3. Rating of medications influence (ROMI): collapsed factors for compliance
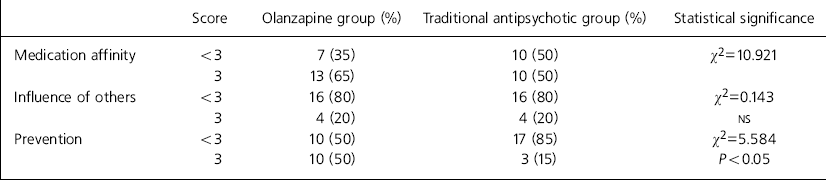
| Score | Olanzapine group (%) | Traditional antipsychotic group (%) | Statistical significance | |
|---|---|---|---|---|
| Medication affinity | <3 | 7 (35) | 10 (50) | χ2=10.921 |
| 3 | 13 (65) | 10 (50) | ||
| Influence of others | <3 | 16 (80) | 16 (80) | χ2=0.143 |
| 3 | 4 (20) | 4 (20) | NS | |
| Prevention | <3 | 10 (50) | 17 (85) | χ2=5.584 |
| 3 | 10 (50) | 3 (15) | P<0.05 |
Comment
No significant difference was found between the two groups with respect to subjective quality of life measures. However, within the traditional antipsychotic group presence of EPS was found to be related to lower affect balance scores. In the LQOLP, the affect balance and self concept scales can be seen as measuring mood and morale, respectively. Both have been shown to influence quality of life outcomes in patients with severe mental illness (Reference OliverOliver, 1991).
More patients taking olanzapine said that they were compliant because they felt their medication stopped their symptoms returning. At the same time there was a trend for patients taking traditional antipsychotics to cite “embarrassment about taking medication” as a potential reason for non-compliance. The reasons for this difference in attitude between the groups are not clear. It may be owing in part to the route of administration rather than the medication itself. The patients were receiving traditional antipsychotic medication in depot form, which might be less acceptable than oral medication for several reasons. These include the need for more frequent contact with services and perceived loss of dignity. Receiving depot medication may also cause physical problems such as painful injection sites. The ‘embarrassment’ cited could also be related to motor dysfunction experienced owing to EPS or akathisia.
Some authors, however, have hinted that improvements in attitude to medication in patients taking olanzapine may be related to more subtle intra-psychic factors. Beasley et al (Reference Beasley, Tollefson and Tran1996b ) found an improvement in quality of life in patients with schizophrenia and that this appeared to be owing to an impact on intra-psychic foundations, a sense of purpose, motivation and emotional interaction. Conversely, although data regarding weight gain in patients taking olanzapine were not available, significant weight gain may have resulted in a negative impact on attitude to medication.
This study provides indirect support for the hypothesis that reduced EPS in patients receiving olanzapine may improve quality of life. However, as patients receiving traditional drugs had been treated for longer than those receiving olanzapine, it is possible that prolonged treatment with olanzapine may also lead to increased rates of EPS with an adverse impact on quality of life. Further, an emerging adverse effect in patients receiving olanzapine is weight gain, which was not assessed in this study. It is possible that this may contribute to self-assessed quality of life in the same way as EPS in those receiving traditional drugs. The study also showed an improvement in attitude to medications in patients taking olanzapine. The reasons for this change in attitude, however, remain unclear. A clear link between the greater tolerability of atypical medications and better compliance rates has yet to be shown, but this study suggests that olanzapine and the newer atypicals represent an advance in the drug treatment of schizophrenia and one that may lead to greater patient satisfaction and, therefore, compliance.






eLetters
No eLetters have been published for this article.