The use of antipsychotics by care home residents has been of concern for many years. In the USA, the prescription of antipsychotics to up to 55% of nursing home residents led to the introduction, in the Omnibus Reconciliation Act 1987, of legal restrictions on the use of these drugs (Reference LEE, GILL and FREEDMANLee et al, 2004). In the UK, antipsychotic prescribing rates to care home residents ranging from 24 to 28% have been reported (Reference MCGRATHMcGrath & Jackson, 1996; Reference PASSMORE, CRAWFORD and BERINGERPassmore et al, 1996; Reference OBORNE, HOOPER and CHI LIOborne et al, 2002; Reference FAHEY, MONTGOMERY and BARNESFahey et al, 2003). Many of the residents of these homes have Alzheimer's disease or other forms of dementia, and treatment of behavioural and psychological symptoms of dementia may be one reason for use of these drugs. However, there is only limited evidence for their efficacy for behavioural and psychological symptoms despite the widespread use (Reference SCHNEIDER and POLLOCKSchneider et al, 1990; Drugs and Therapeutics Bulletin, 2003; Reference LEE, GILL and FREEDMANLee et al, 2004). A recent systematic review concluded that further evidence is required before these drugs can be endorsed (Reference LEE, GILL and FREEDMANLee et al, 2004).
Antipsychotics are associated with significant harm, including an increased risk of falls (Evans, 2003) and long-term cognitive decline (Reference WISNIEWSKI, CONSTANTINIDIS and WEGIELWisniewski et al, 1994; Reference MCSHANE, KEENE and GEDLINGMcShane et al, 1997). After concerns about cardiac safety, the Committee on Safety of Medicines placed restrictions on the prescribing of thioridazine and sertindole; droperidol was discontinued by the manufacturer (Medicines and Healthcare Products Regulatory Agency, 1999, 2000, 2001). An increased risk of ischaemic stroke has been associated with atypical antipsychotic use in elderly patients with dementia (Medicines and Healthcare Products Regulatory Agency, 2004), and this led the Committee on Safety of Medicines to issue guidance that risperidone and olanzapine should not be used for treating behavioural symptoms of dementia. A more recent study suggests that the risk of ischaemic stroke is similar for both atypical and conventional antipsychotics (Reference GILL, ROCHON and HERRMANNGill et al, 2005). Furthermore, typical antipsychotics have been associated with a higher risk of death in the elderly compared with atypicals (Reference WANG, SCHNEEWEISS and AVORNWang et al, 2005).
Prior to its restriction, thioridazine was the most commonly used antipsychotic in UK care settings, accounting for 51-74% of prescriptions (Reference MCGRATHMcGrath & Jackson, 1996; Reference OBORNE, HOOPER and CHI LIOborne et al, 2002). To our knowledge the prescribing of antipsychotics in UK care homes has not been studied since thioridazine was restricted. The aim of this study was to determine the prescribing patterns of antipsychotics in care homes for the elderly since the restrictions on thioridazine.
Method
A cross-sectional study was carried out in 65 care homes for older people in Leeds (nursing, residential and mixed care homes). Baseline data were used from the intervention group of a randomised controlled trial of a clinical medication review of elderly care home residents against usual care (ISRCTN45416155; Reference ZERMANSKY, ALLDRED and PETTYZermansky et al, 2006).
Approval was obtained from the local National Health Service research ethics committee. We approached all care homes in the Leeds area with six or more residents aged 65 and over, seeking to include all residents taking one or more medicines on a long-term basis. We excluded those who were in another clinical trial or who were terminally ill. We excluded individuals at the general practitioner's (GP's) request. We obtained informed consent from those able to grant it and assent from the nearest relative from those with impaired capacity.
Data collection
Clinical data were collected from GP records from 59 practices between April 2002 and July 2003. Details of repeat medicines were recorded for each participant. Typical and atypical antipsychotics were defined as listed in the British National Formulary, section 4.2.1 (British Medical Association & Royal Pharmaceutical Society of Great Britain, 2005).
All participants were assessed for physical dependency using the Barthel Index (Reference WADE and COLLINSWade & Collins, 1988) and cognitive ability using the Standardised Mini-Mental State Examination (SMMSE; Reference MOLLOY and STANDISHMolloy & Standish, 1997).
Results
Prescription data were obtained for the 331 residents in 13 nursing, 38 residential and 14 mixed care homes who were randomised to the intervention group. Care home size ranged from 6 to 128 residents. Different types of homes, for example owner-operated, local authority-owned, medium-sized groups and large chains, were represented. Of the 331 residents, clinical data were available for 320 (97%). Medical records were unavailable for 11 residents owing to death or misplacement of records. Table 1 details residents’ characteristics. There were 146 residents (44%) who had a documented diagnosis of dementia and 249 out of 331 residents (75%) had some degree of cognitive impairment (SMMSE score <23); 24 residents (7%) had a documented history of a psychotic disorder; 82 residents (25%) had a documented medication review by the GP in the previous 12 months.
Table 1. Residents' characteristics (n=331)
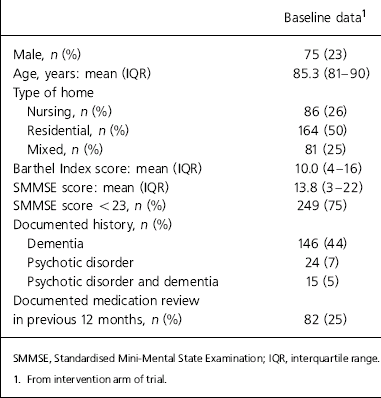
| Baseline data1 | |
|---|---|
| Male, n (%) | 75 (23) |
| Age, years: mean (IQR) | 85.3 (81-90) |
| Type of home | |
| Nursing, n (%) | 86 (26) |
| Residential, n (%) | 164 (50) |
| Mixed, n (%) | 81 (25) |
| Barthel Index score: mean (IQR) | 10.0 (4-16) |
| SMMSE score: mean (IQR) | 13.8 (3-22) |
| SMMSE score <23, n (%) | 249 (75) |
| Documented history, n (%) | |
| Dementia | 146 (44) |
| Psychotic disorder | 24 (7) |
| Psychotic disorder and dementia | 15 (5) |
| Documented medication review in previous 12 months, n (%) | 82 (25) |
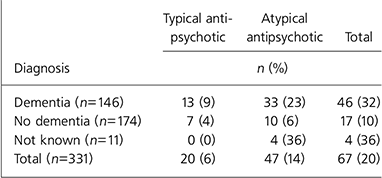
Table 2 details antipsychotic prescribing. Out of 331, 67 residents (20%) were prescribed an antipsychotic, with 47 (14%) and 20 (6%) prescribed an atypical and typical antipsychotic respectively. Of the 67 residents prescribed an antipsychotic, 10 (15%) had a documented diagnosis of a psychotic disorder and 46 (68.7%) had a diagnosis of dementia.
Table 2. Antipsychotic prescribing according to type and diagnosis
| Typical antipsychotic | Atypical antipsychotic | Total | |
|---|---|---|---|
| Diagnosis | n (%) | ||
| Dementia (n=146) | 13 (9) | 33 (23) | 46 (32) |
| No dementia (n=174) | 7 (4) | 10 (6) | 17 (10) |
| Not known (n=11) | 0 (0) | 4 (36) | 4 (36) |
| Total (n=331) | 20 (6) | 47 (14) | 67 (20) |
Of the 146 residents with a diagnosis of dementia, 46 (32%) were prescribed an antipsychotic (typical 13 (9%); atypical 33 (22%)). Only 7 of those prescribed an antipsychotic (10%) had a documented diagnosis of psychotic disorder, although 15 out of 146 of the residents with dementia (10%) had a diagnosis of a psychotic disorder.
In contrast, of the 174 residents without a documented diagnosis of dementia, 17 (10%) were prescribed an antipsychotic (typical 7 (4%); atypical 10 (6%)). Again out of 174, 9 of the residents without dementia (5%) had a diagnosis of a psychotic disorder, but only 3 of these were receiving an antipsychotic. It is not clear why antipsychotics were prescribed to the other 6 residents. For the 11 residents for whom diagnostic information was not available, 4 were prescribed an antipsychotic (all atypical).
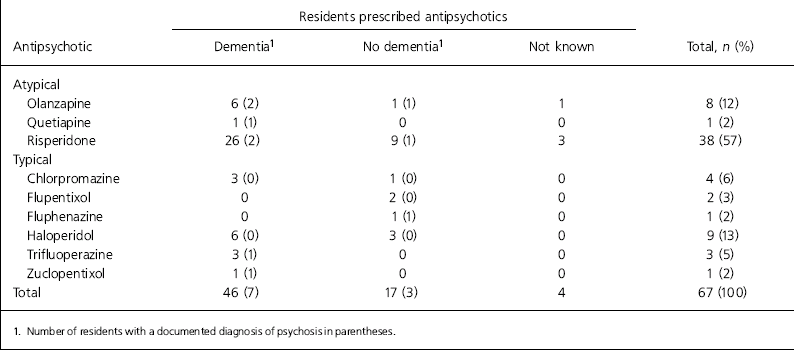
The most commonly prescribed antipsychotic was risperidone (mean dose 1 mg), which accounted for 57% of antipsychotic use (Table 3).
Table 3. Antipsychotic prescribing by drug and documented diagnosis
| Residents prescribed antipsychotics | ||||
|---|---|---|---|---|
| Antipsychotic | Dementia1 | No dementia1 | Not known | Total, n (%) |
| Atypical | ||||
| Olanzapine | 6 (2) | 1 (1) | 1 | 8 (12) |
| Quetiapine | 1 (1) | 0 | 0 | 1 (2) |
| Risperidone | 26 (2) | 9 (1) | 3 | 38 (57) |
| Typical | ||||
| Chlorpromazine | 3 (0) | 1 (0) | 0 | 4 (6) |
| Flupentixol | 0 | 2 (0) | 0 | 2 (3) |
| Fluphenazine | 0 | 1 (1) | 0 | 1 (2) |
| Haloperidol | 6 (0) | 3 (0) | 0 | 9 (13) |
| Trifluoperazine | 3 (1) | 0 | 0 | 3 (5) |
| Zuclopentixol | 1 (1) | 0 | 0 | 1 (2) |
| Total | 46 (7) | 17 (3) | 4 | 67 (100) |
Discussion
To our knowledge, this is the first study of antipsychotic prescribing in UK care homes since the restrictions on thioridazine use in 2000. The results show that in a sample of residents aged 65 and over, from 65 care homes, one-fifth were prescribed an antipsychotic. This figure is slightly lower than previous UK studies, which reported antipsychotic prescribing rates of 24 to 28% (Reference MCGRATHMcGrath & Jackson, 1996; Reference PASSMORE, CRAWFORD and BERINGERPassmore et al, 1996; Reference OBORNE, HOOPER and CHI LIOborne et al, 2002; Reference FAHEY, MONTGOMERY and BARNESFahey et al, 2003). In a recent study in two Canadian long-term care facilities, 21.5% of residents in one facility and 31.3% in the other were prescribed an antipsychotic (Reference HAGEN, ESTHER and IKUTAHagen et al, 2005). The corresponding figure in a sample of 51 nursing homes in Australia was 24.5% (Reference SNOWDEN and DAYSnowden et al, 2006). In our study, almost one-third of residents with a diagnosis of dementia were receiving an antipsychotic. Of those prescribed an anti-psychotic, 15% had a documented diagnosis of a psychotic disorder and it is therefore reasonable to assume that 85% were receiving antipsychotics for unlicensed indications.
In our sample, risperidone had replaced thioridazine as the most commonly prescribed agent and demonstrated a shift from typical to atypical antipsychotic use. The mean dose of risperidone used was 1 mg daily. It seems likely that risperidone became the standard choice of antipsychotic for use in care homes after the restrictions imposed on thioridazine, without any reduction in the overall rate of prescribing. It is possible that since the safety concerns about risperidone and olanzapine have come to light, other antipsychotics may have taken their place. Given the reports that these concerns may be applicable to all antipsychotics (Reference GILL, ROCHON and HERRMANNGill et al, 2005; Reference WANG, SCHNEEWEISS and AVORNWang et al, 2005), it would seem desirable to reduce the frequency of antipsychotic prescribing for residents of care homes, particularly as the majority of residents in this study had evidence of cognitive impairment.
This study was conducted in a randomised sample of approximately 10% of care home residents in Leeds. It included homes of a range of size and type, and covered inner city, suburban and rural areas. However, the results may not be generalisable to care home residents in other locations.
The UK National Service Framework for Older People requires an annual review of all medicines and a 6-monthly review for those on four or more medicines (Department of Health, 2001). However, in this study, only one-quarter of patients had a documented medication review by their GP within the previous 12 months. Antipsychotics can be withdrawn in up to one-half of patients with no adverse effects on behaviour and functioning (Reference AVORN, SOUMERAI and EVERITTAvorn et al, 1992; Reference SCHMIDT, CLAESSON and WESTERHOLMSchmidt et al, 1998), and alternative strategies are effective in reducing agitation in people with dementia (Reference BALLARD, O'BRIEN and REICHELTBallard et al, 2002; Reference HOLMES, HOPKINS and HENSFORDHolmes et al, 2002). Practices should therefore use the medication review markers on electronic records to ensure that all patients and their treatment are reviewed regularly and the review documented. The reviewer should always consider whether it might be appropriate to withdraw medication. Reviews should be conducted by clinicians with an interest in the management of dementia.
Behavioural approaches may be the treatment of choice for the behavioural and psychological symptoms of dementia, but may not be practical for very disturbed behaviour. Furthermore, such expertise and resources are limited in the care home setting. Antipsychotics may therefore be prescribed in desperation in the face of challenging behaviour. However, there is little evidence of the value of antipsychotics in this situation. Furthermore, since this survey, studies have suggested that they may be harmful to physical health (Medicines and Healthcare Products Regulatory Agency, 2004; Reference GILL, ROCHON and HERRMANNGill et al, 2005; Reference WANG, SCHNEEWEISS and AVORNWang et al, 2005).
Declaration of interest
P.B. has given talks for and received hospitality from Jansenn-Cilag.
Acknowledgements
We thank the other members of the trial team, Professor Nick Freemantle and Dr Joanne Eastlaugh, Department of Primary Care and General Practice, University of Birmingham, Mrs Susan Thornton and Mrs Denise Buttress, School of Healthcare, University of Leeds. The study was funded by The Health Foundation.






eLetters
No eLetters have been published for this article.