Published online by Cambridge University Press: 15 September 2014
1. It is pointed out that for very small adsorptions the adsorption law appears to be
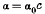
for both gases and solutions.
2. The general form of the adsorption curve for solutions is deduced from the above conclusion and found to agree with the results of different observers.
The author wishes to thank Professor James Walker, F.R.S., for his assistance in the presentation of this paper.
page 48 note * See Ostwald, , Lehrbuch der allgemeinen Chemie (1906), II, iii, p. 253.Google Scholar
page 48 note † Proc. Roy. Soc. (1906), A, 78, p. 9.
page 49 note * Zeits. f. physik. Chem. (1910), lxxiv, p. 641.
page 49 note † Journ. Amer. Chem. Soc. (1917), xxxix, p. 1828.
page 51 note * Kapillarchemie (1909), p. 150.
page 51 note ‡ Zeits. f. physik. Chem. (1914), lxxxvii, p. 669.
page 51 note ‖ Zeits. f. physik. Chem. (1915), xc, p. 681.
page 51 note ** Jour. f. prakt. Chem. (1881), xxiii, p. 324.
page 51 note † Loc. cit., p. 171.
page 51 note § Loc. cit. (1909), lxvii, p. 732.
page 51 note ¶ Loc. cit. (1895), xv, p. 56.
page 51 note †† Trans. Farad. Soc. (1914), x, p. 155.
page 53 note * B.A. Reports, 1911, p. 328.
page 53 note † Zeits. f. physik. Chem. (1910), lxxiv, p. 689.
page 53 note § Medd. f. K. Vet. Akad. Nobelinstitut (1913), ii, 27.
page 53 note ‡ Medd. f. K. Vet. Akad. Nobelinstitut (1916), xci, p. 103
page 54 note * Trans. Farad. Soc. (1914), x, p. 155.
page 54 note † Koll. Zeits. (1914), xiv, p. 242.
page 54 note ‡ Zeits. f. physik. Chem. (1916), xci, p. 385.
page 54 note § Mem. Coll. Sci. Kyoto (1915), i, p. 257.