No CrossRef data available.
Article contents
III.—Observations on the Balmer Series of Hydrogen
Published online by Cambridge University Press: 15 September 2014
Extract
According to the quantum theory of the production of spectra the lines Hα and Hβ of the Balmer series in the hydrogen spectrum may be produced in six and eight different ways respectively. This general conclusion, however, has to be modified. The mass of an electron in its elliptical orbit is not constant but depends upon its velocity. Consequently, the total energy of an electron in its orbit must be expressed in the form
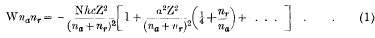
where na and nr are the azimuthal and radial quantum numbers and where 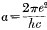 , and the other symbols have their usual meaning.
, and the other symbols have their usual meaning.
- Type
- Proceedings
- Information
- Copyright
- Copyright © Royal Society of Edinburgh 1925
References
page 15 note * Proc. Roy. Soc. Edin., vol. xliii, part i, p. 37.
page 18 note * Proc. Roy. Soc., A, vol. xcvii, p. 307.
page 18 note † Phil. Mag., vol. xxix, p. 274.


