Published online by Cambridge University Press: 05 December 2011
Photogeneration of singlet oxygen molecules (1O2), their vibrationally excited state 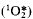 and dimols (1O2)2 has been shown by measuring photosensitised delayed luminescence in pigment-containing media. All singlet oxygen species are formed as a result of energy transfer to O2 from triplet pigment molecules. Monomeric pigment molecules are the most efficient singlet oxygen generators. The 1O2 quantum yields are 40–80% in aerobic solutions of monomeric chlorophylls and pheophytins. Pigment aggregation causes a strong decrease in singlet oxygen production. The 1O2 quantum yield in chloroplasts has been estimated using literature and experimental data on formation of the chlorophyll triplet states in the photosynthetic apparatus. The most probable value is 0.1%. One of the major sources of singlet oxygen is likely to be the triplet states of newly formed pigment molecules which are not quenched by carotenoids and can be detected by measuring low-temperature pigment phosphorescence. Quenching of singlet oxygen by the thylakoid components has been analysed and the 1O2 lifetime estimated. The data suggest that carotenoids and chlorophylls are the most efficient physical 1O2 quenchers and the 1O2 lifetime is about 70 ns in thylakoids. The quantum yield of 1O2-induced pigment photodestruction was estimated to be about 10−6–10−5. This value is close to the quantum yield of chlorophyll photobleaching experimentally observed in aerobic suspensions of isolated chloroplasts. The intensity of pigment phosphorescence at 77 K correlates with the rate of chlorophyll photobleaching in plant materials. The data suggest that 1O2 generation by the pigment triplet states is the most likely reason for chloroplast photodamage. The intensity of pigment phosphorescence can be used as an index of the degree of plant photo-oxidative stress.
and dimols (1O2)2 has been shown by measuring photosensitised delayed luminescence in pigment-containing media. All singlet oxygen species are formed as a result of energy transfer to O2 from triplet pigment molecules. Monomeric pigment molecules are the most efficient singlet oxygen generators. The 1O2 quantum yields are 40–80% in aerobic solutions of monomeric chlorophylls and pheophytins. Pigment aggregation causes a strong decrease in singlet oxygen production. The 1O2 quantum yield in chloroplasts has been estimated using literature and experimental data on formation of the chlorophyll triplet states in the photosynthetic apparatus. The most probable value is 0.1%. One of the major sources of singlet oxygen is likely to be the triplet states of newly formed pigment molecules which are not quenched by carotenoids and can be detected by measuring low-temperature pigment phosphorescence. Quenching of singlet oxygen by the thylakoid components has been analysed and the 1O2 lifetime estimated. The data suggest that carotenoids and chlorophylls are the most efficient physical 1O2 quenchers and the 1O2 lifetime is about 70 ns in thylakoids. The quantum yield of 1O2-induced pigment photodestruction was estimated to be about 10−6–10−5. This value is close to the quantum yield of chlorophyll photobleaching experimentally observed in aerobic suspensions of isolated chloroplasts. The intensity of pigment phosphorescence at 77 K correlates with the rate of chlorophyll photobleaching in plant materials. The data suggest that 1O2 generation by the pigment triplet states is the most likely reason for chloroplast photodamage. The intensity of pigment phosphorescence can be used as an index of the degree of plant photo-oxidative stress.