Published online by Cambridge University Press: 05 December 2011
Salix has many physiological features in common with other deciduous woody plants, e.g. C3 photosynthesis, occurrence of latitudinal photoperiodic ecotypes, and organic N (no 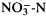 ) flux to the shoot in the xylem. Special points about the physiology of Salix spp. which may have impact on their ecology and economic uses include: (i) relatively high (for woody plants) light-saturated rate of photosynthesis on a leaf area or leaf dry weight basis, (ii) sex differences in water (transpiration) costs of growth, (iii) very limited seed longevity and a wide range of temperature and light conditions permitting germination, and (iv) ready rooting and establishment of naturally or artificially detached twigs and branches. Areas in which work on Salix has been especially influential for the development of plant physiology include: (i) the analysis of phloem functioning using aphids, (ii) the role of photoinhibition under natural conditions, and (iii) the realisation that the woody habit need not constrain the achieved activity of enzymes and hence N-based metabolic rates.
) flux to the shoot in the xylem. Special points about the physiology of Salix spp. which may have impact on their ecology and economic uses include: (i) relatively high (for woody plants) light-saturated rate of photosynthesis on a leaf area or leaf dry weight basis, (ii) sex differences in water (transpiration) costs of growth, (iii) very limited seed longevity and a wide range of temperature and light conditions permitting germination, and (iv) ready rooting and establishment of naturally or artificially detached twigs and branches. Areas in which work on Salix has been especially influential for the development of plant physiology include: (i) the analysis of phloem functioning using aphids, (ii) the role of photoinhibition under natural conditions, and (iii) the realisation that the woody habit need not constrain the achieved activity of enzymes and hence N-based metabolic rates.