Published online by Cambridge University Press: 05 December 2011
The parents of the three naturally occurring radioactive decay series (text-fig. 1),232Th, 238U and 235U, have existed since the time of formation of the earth and 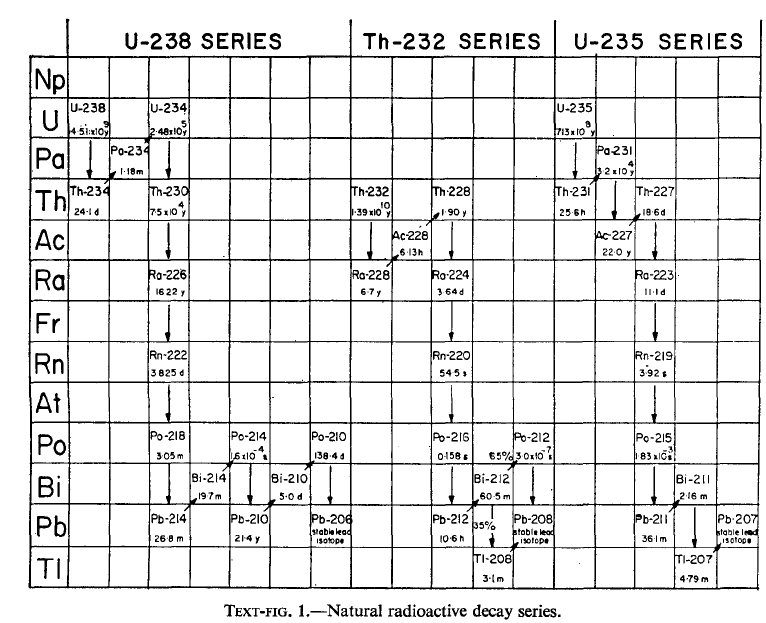 through the process of radioactive decay have continuously generated their shorterlived daughter radio-isotopes. Under conditions where these decay products are not separated from the parents the situation referred to as secular equilibrium may be attained at which the activity ratio of any two daughters in the same decay chain is unity. The time required for the attainment of this situation corresponds to several half-lives of the longest lived daughter nuclide. In a great many instances, however, secular equilibrium is not achieved. Excellent examples of disequilibrium are to be found in the distribution of natural radioactive decay series elements in the oceans and sediments. These situations can be used to advantage in marine geochemistry to obtain information on residence times of elements in the oceans and rates of sedimentation occurring under a variety of conditions.
through the process of radioactive decay have continuously generated their shorterlived daughter radio-isotopes. Under conditions where these decay products are not separated from the parents the situation referred to as secular equilibrium may be attained at which the activity ratio of any two daughters in the same decay chain is unity. The time required for the attainment of this situation corresponds to several half-lives of the longest lived daughter nuclide. In a great many instances, however, secular equilibrium is not achieved. Excellent examples of disequilibrium are to be found in the distribution of natural radioactive decay series elements in the oceans and sediments. These situations can be used to advantage in marine geochemistry to obtain information on residence times of elements in the oceans and rates of sedimentation occurring under a variety of conditions.