Background
Global population ageing
Unprecedented technological developments coupled with advances in medicine and public health over the past two centuries has contributed to exponential growth in the global population from 1 billion in 1800, to a projected 8 billion in 2023(Reference Mack1–Reference Cutler and Miller3). This population growth can be attributed to profound reductions in child mortality and the rapid increase in life expectancy from 40 to 80 years in developed countries(Reference Christensen, Doblhammer and Rau4–6). The extension in life expectancy has resulted in an increasing population of older adults, a situation exacerbated by lower fertility rates, especially in developed countries(Reference Population and Fertility7). In 2018, for the first time in human history, the number of people aged ≥65 years surpassed those aged ≤5 years of age globally(8). The combination of these two demographic phenomena has resulted in a growing, yet increasingly ageing population throughout the developed world; with similar patterns emerging in the developing world(Reference Population and Fertility7). By 2030, one-in-six Europeans will be aged over 60 years and by 2040 one-in-four older adults will be aged over 85 years(9). This global phenomenon of population ageing presents pressing challenges to the sustainability of our healthcare, social care and welfare systems, particularly if increases in lifespan are decoupled from increases in healthspan.
Lifespan and healthspan
Increases in the total number of years lived (lifespan) are not keeping pace with gains in the number of years lived free of disease and disability (healthspan). The gap between lifespan and healthspan is estimated at 9 years(10). More pessimistic estimates suggest that as much as one-fifth of life may be lived with chronic disease(Reference Partridge, Deelen and Slagboom11). Chronic conditions of mid- and later-life account for 4 out of every 5 years lived with disability, while four conditions – CVD, cancer, diabetes and respiratory diseases – account for 80 % of chronic disease-related deaths(12,Reference Cheng, Yang and Schwebel13) .
Current health and social care strategies are largely ineffective in closing the gap between lifespan and healthspan. The increasing demand on healthcare and social support services resulting from living longer, with a growing burden of disease and disability, is becoming ever more apparent to governments, policy makers, service planners and stakeholders.
This is underscored by high profile programmes targeting the extension of healthy lifespan such as the United Nations Sustainable Development Goals and the WHO Decade of Healthy Ageing. To achieve measurable gains in healthspan, thereby sustaining the ageing population, a more coordinated and cohesive approach across medicine, science, government and wider civic society will be necessary(Reference Garmany, Yamada and Terzic14). Fundamental to this will be targeting modifiable risk and ameliorating factors that are useful in terms of preventing, monitoring and intervening in the onset and progression of age-related conditions.
Biological v. chronological age
Chronological age is associated with declines in physical and cognitive health, the risk of adverse health outcomes and mortality, and the increased use of health and social care services(Reference Cheng, Yang and Schwebel13,Reference Beard, Officer and de Carvalho15) . However, older adults of the same age are not at the same risk for these outcomes(Reference Hamczyk, Nevado and Barettino16). Ageing is a dynamic and heterogeneous process and there is substantial variability in how we age biologically. Biological ageing occurs due to damage and dysregulation at the cellular (macromolecules and cells), physiological (tissue, organ, system) and functional (organismal) level, ultimately manifesting in the decline of physical and cognitive function(Reference Ferrucci, Levine and Kuo17,Reference Ferrucci, Gonzalez-Freire and Fabbri18) . Indeed, the heterogeneity in the pace of biological ageing becomes more pronounced, with more divergent trajectories, at older ages(Reference Ferrucci, Levine and Kuo17,Reference Lowsky, Olshansky and Bhattacharya19) . During the recent coronavirus disease (COVID-19) pandemic, many countries advised adults aged ≥70 years to shield to prevent infection and mortality and protect health services(Reference Mueller, McNamara and Sinclair20). However, using chronological age to characterise individual mortality risk as the basis to implement a blanket policy for older adults at the population level had its problems. Such policies did not take account of the heterogeneity in the pace of ageing among older adults and failed to recognise the consequent harms of physical deconditioning, social isolation, loneliness, depression and decreased quality of life(Reference Briggs, McDowell and De Looze21–Reference McGarrigle, Ward and De Looze23).
In this narrative review, we will focus on the potential value of micronutrients and maintaining micronutrient sufficiency, to sustaining the health of the ageing population. We will pay particular attention to two common and interlinked conditions of ageing, frailty and cognitive impairment, which significantly impact the ability of older adults to sustain functional capacity and independence. The graphical abstract depicts the role of micronutrient status and the physiological systems that underpin frailty and cognitive impairment.
Frailty and cognitive decline in older adults
Frailty and the disability cascade
As discussed earlier, not everyone of the same age is at the same risk of adverse health outcomes. Frailty captures this differential biological risk that is distinct from, but related to, chronological ageing(Reference Clegg, Young and Iliffe24). It is a common condition in older adults, although it is not an inevitable part of ageing. While recognised as a clinical syndrome, frailty is not a medical diagnosis because it can have different underlying causes in different individuals. Frailty is characterised by multisystem loss of physiological reserve, systemic decompensation in response to stressors (e.g. infection, medication change or a change in living arrangements) and increased risk of adverse outcomes including falls, disability and mortality, independently of chronological age(Reference Howlett, Rutenberg and Rockwood25). It is also predictive of increased use of health and social care services(Reference Roe, Normand and Wren26). Frailty is a dynamic process that can be viewed on a continuum. An older person can transition in either direction between robustness or non-frailty, pre-frailty (an intermediate sub-clinical state) and frailty(Reference O'Halloran, Hartley and Moloney27–Reference Romero-Ortuno, Hartley and Davis29). Thus, it can represent a transition between healthy ageing and disability, and is a target condition for the prevention of disability and the extension of healthy life years(Reference Dent, Martin and Bergman30–Reference Hoogendijk, Afilalo and Ensrud32).
The gold standard methodology for the assessment and management of frailty is comprehensive geriatric assessment. Comprehensive geriatric assessment is a holistic and interdisciplinary assessment of the individual, resulting in a personalised care plan, and has been demonstrated to reduce the risk of disability, cognitive decline, long-term residential care and death(Reference Ellis, Gardner and Tsiachristas33,Reference van Rijn, Suijker and Bol34) . However, this approach is unfeasible for systematic case finding at the population level. The frailty phenotype(Reference Fried, Ferrucci and Darer35,Reference Fried, Tangen and Walston36) , the frailty index(Reference Mitnitski, Song and Rockwood37,Reference Rockwood and Mitnitski38) and the clinical frailty scale(Reference Rockwood, Song and MacKnight39,Reference Theou, Perez-Zepeda and van der Valk40) are widely accepted screening instruments for diagnosing frailty at the population level. The choice of frailty instrument depends largely on the type of clinical or research setting, the participant or patient group, availability of trained personnel, time constraints and administrative burden(Reference Martin and O'Halloran41). Internationally, the prevalence of frailty is 4–59 % among adults aged ≥65 years, depending on the frailty instrument applied and population under study(Reference Collard, Boter and Schoevers42).
Associations between frailty and cognitive impairment
The study of the relationship between frailty and cognitive decline is complex with several different approaches considered including the examination of effects of separate domains on one another, temporal studies and bidirectionality.
Physical function (often as individual components of the frailty phenotype) and its association with cognitive impairment have been examined. For example, slow gait speed(Reference Yassuda, Lopes and Cachioni43) and a decline in grip strength(Reference Sternäng, Reynolds and Finkel44) have been linked to cognitive impairment and poorer performance on tests of memory, verbal skills, spatial skills and processing speed, respectively. Further, some studies have investigated the temporal relationship between the reduction of muscle strength and cognitive ability, suggesting that cognitive decline may precede physical decline(Reference Inzitari, Baldereschi and Carlo45,Reference Taekema, Ling and Kurrle46) , although the evidence suggests that this effect is attenuated when other comorbidities are considered(Reference Atkinson, Rosano and Simonsick47).
While a number of studies have linked frailty to cognitive decline(Reference Mitnitski, Fallah and Rockwood48), with some indicating frailty predicts global cognitive decline and incident Alzheimer's disease(Reference Buchman, Boyle and Wilson49), many investigations have focused on the associations between frailty and individual domains of cognitive function. In a recent longitudinal study, participants who were frail showed deficits on assessments of verbal fluency and information processing speed over a 12-year period(Reference Bunce, Batterham and Mackinnon50).
Current evidence suggests that worsening frailty among older adults is considered a precursor to cognitive impairment, to a lesser extent, the reverse may also be true(Reference Yassuda, Lopes and Cachioni43,Reference Sternäng, Reynolds and Finkel44) . Frailty and cognitive impairment have a variety of underlying causes, both conditions can predict incident dementia, and each may influence the other. As they are highly correlated with advancing age, it is expected that the two will interact as people age(Reference Inzitari, Baldereschi and Carlo45,Reference Taekema, Ling and Kurrle46) . Understanding their co-occurrence and interplay could therefore shed light on pathophysiology, management and prevention. The combination of two perspectives that are typically treated individually presents a challenge in the study of frailty and cognitive impairment. Additionally, only a few studies have explicitly investigated frailty and cognitive impairment in this manner. Although frail older persons may perform poorly on cognitive tests, they may not show significant changes in the cognitive tests, according to research that looked at the bidirectionality of frailty and individual domains of cognitive function(Reference Bunce, Batterham and Mackinnon50,Reference Ávila-Funes, Amieva and Barberger-Gateau51) . Conversely, some researchers have examined a bidirectional association between components of frailty indices, namely physical function, and cognition. A significant predictive value of baseline handgrip strength on the onset of further cognitive decline was recently confirmed by a longitudinal study conducted on an American population over a 20-year period(Reference McGrath, Vincent and Hackney52). Interestingly, the authors also highlighted a significant bidirectional relationship in which the absence of cognitive deficit or the presence of increasing baseline cognitive deficit severity related to progressively higher risks of weaker handgrip strength(Reference McGrath, Vincent and Hackney52). The consistent bidirectional association between physical and cognitive functions has also been validated in a large Korean population over 8 years, with results suggesting that these conditions might share common pathways such as oxidative stress or chronic inflammation(Reference Kim, Sun and Han53). Oxidative stress(Reference Mulero, Zafrilla and Martinez-Cacha54) and chronic inflammation(Reference Panza, Seripa and Solfrizzi55) are associated with both frailty and with cognitive decline.
No matter the method of measurement, frailty is a broader, more comprehensive concept that incorporates deficits across various domains and incorporates many aspects of physical function. Thus, rather than focusing solely on physical function, using the concept of frailty allows for the assessment of a more comprehensive measure of health and susceptibility. Few longitudinal studies have explored a bidirectional relationship between frailty and cognitive impairment. One such study by Godin et al.(Reference Godin, Armstrong and Rockwood56) identified a significant bi-directional relationship across two waves of SHARE, a study based in Europe.
Cognitive frailty
Another strategy for the exploration of the relationship between frailty and cognitive impairment is the concept of cognitive frailty. Defined by the International Academy of Nutrition and Aging/International Association of Gerontology and Geriatrics as ‘the simultaneous presence of physical frailty operationalized with the frailty phenotype model and cognitive impairment diagnosed with a CDR score of 0⋅5 among older adults without concurrent Alzheimer's disease (AD) or any other form of dementia’, it is used to characterise people who have both features but have not been clinically diagnosed with dementia(Reference Kelaiditi, Cesari and Canevelli57). The inclusion of cognitive measures in the assessment of frailty can improve the predictive validity of the phenotype regarding unfavourable health and is important for assessing both physical and cognitive function in older adults for the planning of timely interventions.
The role of nutrition in frailty and cognitive impairment
Nutrition and frailty are intrinsically linked. Unintentional weight loss is a key susceptibility factor for frailty, according to Fried et al. (Reference Fried, Tangen and Walston36). Primary sarcopenia, due in part to macro- and micro-nutrient deficiencies, is common among frail populations(Reference Bollwein, Volkert and Diekmann58,Reference Lorenzo-López, Maseda and de Labra59) with up to 90 % of older persons who are malnourished also being more frail(Reference Bollwein, Volkert and Diekmann58,Reference Verlaan, Aspray and Bauer60,Reference Batsis and Villareal61) .
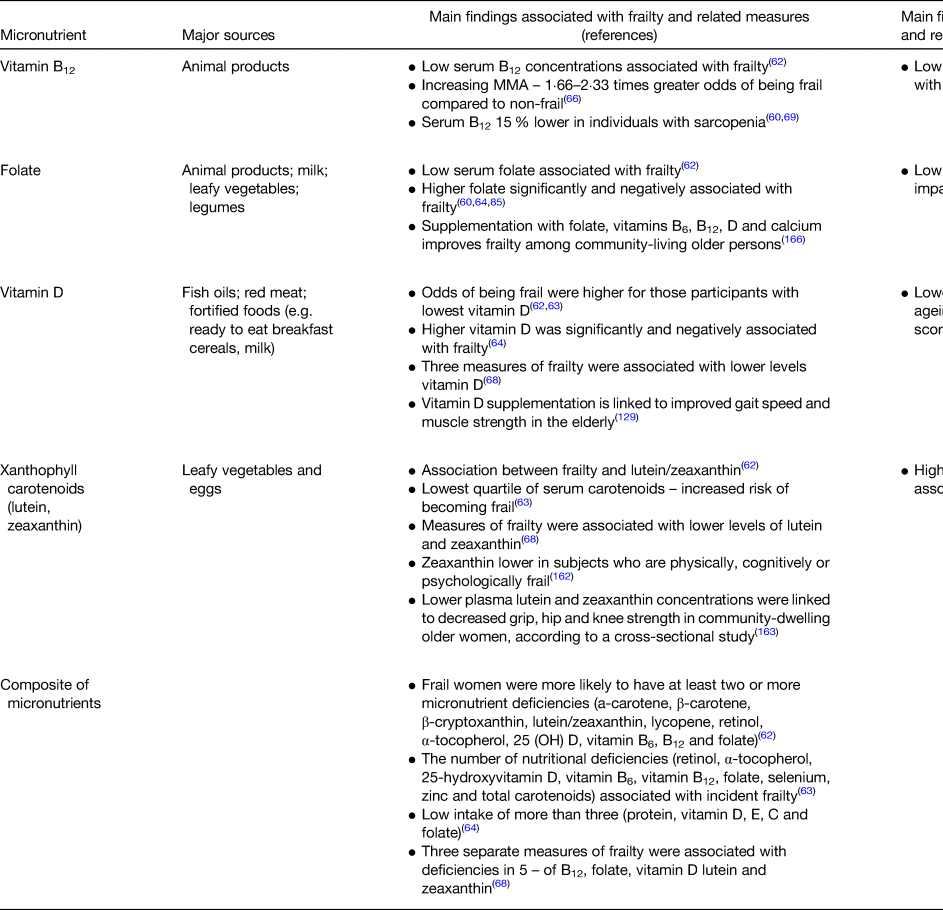
While many studies have focused on the impact of macronutrient (e.g. protein) intake on frailty, fewer have reported associations between explicit frailty and the circulating micronutrients captured within the scope of this review(Reference Michelon, Blaum and Semba62–Reference O'Halloran, Laird and Feeney68) (Table 1). Previous investigations have exhibited design limitations including modest or female-only or relatively young samples or have used a single frailty measure(Reference Michelon, Blaum and Semba62–Reference Smit, Winters-Stone and Loprinzi67). However, a study from our research group used a large representative sample of adults aged 50 and over, to demonstrate that lower levels of lutein, zeaxanthin and vitamin D were associated with three different measures of frailty, and also that these relationships were evident in measures of prefrailty (Fig. 1)(Reference O'Halloran, Laird and Feeney68).

Fig. 1. Micronutrient insufficiency associated with pre-frailty and frailty as indexed by the frailty phenotype and frailty index. Adapted from O'Halloran et al., J Am Med Dir Assoc. 2020.
Table 1. Summary of the existing relationship between selected micronutrients, frailty and cognitive impairment
Nutrition is a modifiable risk factor that has been associated with many non-communicable diseases linked to dementia, such as diabetes and CVD(Reference Riederer, Korczyn and Ali69,Reference Santos, Snyder and Wu70) . Lifelong nutrition may also have a direct effect on brain function, for example, longitudinal studies have reported associations between certain dietary patterns or nutrients and brain-volume loss(Reference Hooshmand, Mangialasche and Kalpouzos71,Reference Luciano, Corley and Cox72) and integrity(Reference Virtanen, Siscovick and Lemaitre73), with some clinical trials confirming these results(Reference Witte, Kerti and Margulies74,Reference Smith, Smith and De Jager75) . Oxidative stress is thought to be a major contributor to neurodegeneration and depression(Reference Bishop, Lu and Yankner76); thus, antioxidants such as vitamin C(Reference Hamer, Bates and Mishra77–Reference Luchsinger, Tang and Shea80), E(Reference Banikazemi, Mokhber and Safarian81) and β-carotene may be important(Reference Engelhart, Geerlings and Ruitenberg82), but no clear conclusions can be made. Overall, a substantial body of research, largely from observational studies, points to a direct impact of lifelong nutrition on clinical indicators of cognitive status in older persons.
Vitamin B12 and folate
The role of vitamin B12 and folate in frailty
Vitamin B12 and folate have been linked to various chronic diseases of ageing such as CVD, diabetes and cognitive impairment(Reference Bailey83,Reference Kennedy84) . These water-soluble micronutrients are essential co-factors in one-carbon metabolism, DNA-methylation and nucleotide synthesis, serving a regulatory effect in all tissues in the body, including systemic inflammation(Reference Kennedy84). Therefore, it has been proposed that these B vitamins provide a fundamental framework for comprehending the onset and development of frailty, by their modulating effect on cellular processes. Deficiency or low serum levels of vitamin B12 (<400 pg/ml, equivalent to <295⋅1 pm/l) seem to have a negative effect on conditions such as sarcopenia(Reference Bulut, Soysal and Aydin85) and other musculoskeletal disorders(Reference Pannérec, Migliavacca and De Castro86) related to frailty. Conversely, it has been observed that ageing and frailty can lead to vitamin B12 deficiency(Reference Verlaan, Aspray and Bauer60). Low folate has also been independently associated with frailty(Reference Michelon, Blaum and Semba62,Reference Bartali, Frongillo and Bandinelli64) , but this relationship has not been observed consistently(Reference Smit, Winters-Stone and Loprinzi67,Reference O'Halloran, Laird and Feeney68) .
The role of vitamin B12 and folate in cognitive impairment
B-vitamins that are involved in one-carbon metabolic pathways have been studied extensively for their potential effect on cognitive impairment and dementia(Reference Smith and Refsum87). Both vitamin B12 and folate, in addition to vitamin B6 and riboflavin, are required for DNA synthesis and repair, amino acid metabolism and methylation reactions. Further, these B-vitamins are required for efficient metabolism of homocysteine (HCY), a cytotoxic intermediary amino acid that is a downstream product of biological methylation reactions(Reference Smith and Refsum87). Observational evidence suggests that the inhibition of methylation reactions may influence cognitive impairment in ageing(Reference Smith and Refsum87) and there is growing interest in the possibility that a loss of cognitive function may partly be explained by inadequate status of these vitamins(Reference Raman, Tatsioni and Chung88–Reference Vogel, Dali-Youcef and Kaltenbach91). Severe vitamin B12 deficiency, such as that seen in pernicious anaemia, causes severe neurological consequences including sensory and motor neuropathy. Low or deficient vitamin B12 status is associated with depression(Reference Laird, O'Halloran and Molloy92) and altered mental status and cognitive decline(Reference Green, Allen and Bjørke-Monsen93). It also reduces the availability of folate for DNA synthesis(Reference Green, Allen and Bjørke-Monsen93). Age-related deficiencies in folate transport and metabolism, use of anti-folate drugs, genetic factors and excessive alcohol consumption are among the factors that contribute to vitamin B12 and folate insufficiency(Reference Allen94), frequently seen in older persons(Reference Clarke, Grimley Evans and Schneede95). In Irish longitudinal study on ageing (TILDA), the prevalence of older people with deficient or low vitamin B12 (<185 pm/l) and folate (<10 nm/l) status was 12 % and 15 %, respectively(Reference Laird, O'Halloran and Carey96).
Several epidemiological studies have shown cross-sectional and prospective associations between low vitamin B12(Reference Green, Allen and Bjørke-Monsen93) and folate(Reference Quadri, Fragiacomo and Pezzati97–Reference Fu, Liu and Zhu106), and the risk of cognitive impairment and dementia. As demonstrated previously by our group(Reference O'Connor, Scarlett and De Looze107), low baseline folate levels can predict a reduction in overall cognitive function and episodic memory in older persons who were cognitively healthy, making them a potential key marker for the risk of early decline (Fig. 2). This was consistent with other studies showing low folate status was associated with higher risks of cognitive impairment or dementia(Reference Quadri, Fragiacomo and Pezzati97–Reference Fu, Liu and Zhu106).

Fig. 2. Low folate predicts cognitive decline over 8 years. Adapted from O'Conner et al., Eur J Clin Nutr. 2022.
In addition, selected observational data have suggested that older adults with simultaneous low vitamin B12 and high folate status had higher risks of anaemia and cognitive impairment or decline(Reference Morris, Jacques and Rosenberg108–Reference Moore, Ames and Mander110), given that high-dose folic acid (FA) treatment was shown to temporarily mask clinical symptoms in persons with pernicious anaemia(Reference Ross, Belding and Paegel111). However, the causal relevance of these associations remains uncertain with conflicting results(Reference Clarke, Sherliker and Hin112–Reference Doets, Ueland and Tell114). TILDA reported that those with low B12 combined with high folate status did not have any adverse associations with cognitive performance compared. In contrast, the study demonstrated that higher concentrations of folate were associated with small, but statistically significant higher scores for global cognitive performance in this setting(Reference O'Connor, Laird and Carey115).
Evidence from randomised controlled trials (RCT) of B-vitamins has shown no consistent benefit of supplementation on cognitive outcomes. FA supplementation was associated with improved domain-specific cognitive performance in RCT with relatively large samples and ≥2 years follow-up(Reference de Jager, Oulhaj and Jacoby116–Reference Walker, Batterham and Mackinnon118). One trial that examined individuals with high HCY to exclude causes other than low folate concentrations and FA supplementation (0⋅8 mg oral FA daily) was associated with improved memory, processing speed and sensorimotor speed after 3 years(Reference Durga, van Boxtel and Schouten117). Supplementation was more effective in improving processing speed in those with high baseline HCY levels (>12⋅9 μm/l) and in improving information processing and sensorimotor speed in those with low baseline vitamin B12 concentrations (<250 pm/l)(Reference Durga, van Boxtel and Schouten117).
In another trial, testing combinations of folate, vitamins B6 and B12, and n-3 fatty acids for 4 years were effective in preserving semantic memory or temporal orientation in a subgroup of participants with previous coronary artery disease or ischaemic stroke, but not in the total trial population of 1748 men and women aged 45–80 years(Reference Andreeva, Kesse-Guyot and Barberger-Gateau119). These observations suggest that individuals with high baseline HCY, low baseline vitamin B concentrations or established cardiovascular and cerebrovascular disease might benefit most from vitamin B supplementation.
While most trials that have been carried out have been underpinned by the hypothesis that lowering HCY, which is associated with cognitive impairment, by B-vitamin supplementation, it is plausible that biological mechanisms other than hyperhomocysteinaemia may underlie the associations between B-vitamins and cognitive impairment. Other proposed mechanisms include impaired methylation and misincorporation of uracil into DNA. Vitamins and nutrients often function as a collection of cofactors, therefore interventions using singular or closely related compounds may have too narrow a focus and do not account for the complexity of the synergistic interactions between nutrients. This is illustrated by another trial using FA, B6 and B12, showing treatment was effective only in those with high baseline n-3 fatty acid concentrations. In fact, n-3 fatty acid status was protective against brain atrophy only in the presence of B-vitamin supplementation, suggesting that both are needed for effectiveness(Reference Jernerén, Elshorbagy and Oulhaj120).
Vitamin D
The role of vitamin D in frailty
Due to its well-established relationship with bone and muscle health, vitamin D intake is vital for the ageing population. Vitamin D is known to regulate calcium homeostasis, bone mineralisation and inflammatory response. Vitamin D deficiency (25(OH)D < 30 nm/l) has been consistently reported to be highly prevalent in older adults(Reference Ju, Lee and Kim121) with data from TILDA suggesting a prevalence of 13 %(Reference Laird, O'Halloran and Carey122). Low vitamin D also has been consistently associated with frailty(Reference Michelon, Blaum and Semba62–Reference Bartali, Frongillo and Bandinelli64) and prefrailty in the TILDA cohort (Fig. 1)(Reference O'Halloran, Laird and Feeney68) Evidence linking low vitamin D levels and incident phenotype frailty has been shown in both meta- and longitudinal analyses(Reference Buta, Choudhury and Xue123–Reference Wong, McCaul and Yeap128). After a 3-year follow-up, Vogt et al. observed that participants (>65 years) with baseline vitamin D levels <37⋅5 nm/l, compared with ≥75 nm/l, were more likely to become pre-frail or frail(Reference Vogt, Decke and de Las Heras Gala127). A meta-analysis revealed that vitamin D supplementation is linked to improved gait speed and muscle strength in the older persons(Reference Muir and Montero-Odasso129). Similar to this, a meta-analysis of intervention trials reported that calcium and vitamin D supplementation may help prevent fractures in older persons(Reference Kelaiditi, Cesari and Canevelli57). In addition, vitamin D supplementation was associated with increased global DNA methylation levels and reduced epigenetic ageing(Reference Chen, Dong and Bhagatwala130,Reference Zhu, Bhagatwala and Huang131) . However, the exact role of vitamin D intake in older adults remains unclear, in part due to limitations in intervention study design and targeting of appropriate populations.
Frailty, COVID-19 and vitamin D
The presence of co-morbidity and frailty in older adults has been associated with a higher risk of undesired outcomes and mortality due to COVID-19 and poorer response to COVID-19 vaccination(Reference Hussien, Nastasa and Apetrii132). Therefore, the identification of potentially accessible and low-cost health and lifestyle behaviours that could attenuate this risk in those with frailty remains a high priority.
Recent research has highlighted that vitamin D may have an important function within the immune system. Expression of the vitamin D receptor has been identified on a variety of cells of the immune system including macrophages, T lymphocytes, dendritic cells and monocytes and may act as a modulator through its ability to alter cytokine secretion(Reference Martens, Gysemans and Verstuyf133). For instance, low vitamin D status has been previously associated with markers of inflammation and an enhanced pro-inflammatory profile in older Irish adults(Reference Laird, McNulty and Ward134). Pro-inflammatory cytokines have been implicated in increased severity of COVID-19 and positive modulation of these interleukins by vitamin D has been hypothesised(Reference Laird, Rhodes and Kenny135). Early observational evidence suggested that countries with either a programme of mandatory vitamin D food fortification or higher exposure to UVB vitamin D forming light had lower incidence of COVID-19 and death rates in comparison to countries without fortification or low light exposure(Reference Laird, Rhodes and Kenny135,Reference Rhodes, Subramanian and Laird136) . Actual vitamin D intervention studies have produced mixed results with little to no effect in healthy populations but positive effects in the at-risk frail populations(Reference Annweiler, Corvaisier and Gautier137).
The role of vitamin D in cognitive impairment
The body of evidence for the function of vitamin D in maintaining brain health has been growing since the discovery of the vitamin D receptor in the brain(Reference Eyles, Smith and Kinobe138). Several different neurobiological pathways have been linked(Reference Anastasiou, Yannakoulia and Scarmeas139). A meta-analysis(Reference Jayedi, Rashidy-Pour and Shab-Bidar140) observed an inverse dose–response relationship between the concentrations of vitamin D and risk of dementia or Alzheimer's disease.
Systematic reviews and meta-analyses have demonstrated that Alzheimer's disease patients' serum vitamin D status is lower than that of healthy controls, and that this is related to worse cognitive results(Reference Annweiler, Llewellyn and Beauchet141,Reference Van der Schaft, Koek and Dijkstra142) . Reduced vitamin D status has been linked to faster cognitive ageing and worsening cognitive test scores, according to longitudinal studies(Reference Miller, Harvey and Beckett143,Reference Toffanello, Coin and Perissinotto144) . In addition, Hooshmand et al. used MRI to show that having more vitamin D was linked to larger brain volumes(Reference Hooshmand, Lökk and Solomon145).
Large cross-sectional and prospective investigations revealed that a higher risk of depression was associated with decreased serum vitamin D status(Reference Williams, Sink and Tooze146–Reference Briggs, McCarroll and O'Halloran149). A thorough systematic analysis that incorporated data from cross-sectional, prospective and RCT studies concluded that having reduced vitamin D status may increase the chance of developing late-life depression(Reference Okereke and Singh147). More recently, an extensive meta-analysis of 41 RCT (n53 235) found that vitamin D supplementation reduced the occurrence of depressive symptoms(Reference Mikola, Marx and Lane150). However, experimental evidence of the effect of vitamin D supplementation is scarce, with a recent review suggesting that a role for vitamin D supplementation in enhancing cognition (separate from depression) in adults cannot be supported based on evidence to date(Reference Beauchet, Cooper-Brown and Allali151). The variability of vitamin D concentrations, cognitive tests used, supplementation doses and the samples' characteristics (i.e. ethnicity or number of participants who are deficient) may explain the ambiguity in the findings.
Lutein and zeaxanthin
The role of lutein and zeaxanthin in frailty
Xanthophyll carotenoids have long been implicated in improving visual outcomes and disease progression in individuals with age-related macular degeneration. More recently, a putative protective role for these compounds in other chronic diseases of ageing has emerged, including cancer(Reference Leoncini, Nedovic and Panic152), CVD(Reference Hak, Stampfer and Campos153), diabetes(Reference Hamer and Chida154), neurodegenerative disease(Reference Amadieu, Lefevre-Arbogast and Delcourt155) and bone health(Reference Sugiura, Nakamura and Ogawa156). Citrus fruits, spinach, kale, broccoli, maize and other vegetables and fruits are the main sources of lutein and zeaxanthin in the diet(Reference Perry, Rasmussen and Johnson157). The biological mechanisms underpinning these associations may lie in their antioxidant(Reference Bohn158) and anti-inflammatory properties(Reference Ben-Dor, Steiner and Gheber159,Reference Palozza, Simone and Catalano160) and the promotion of cell membrane stabilisation(Reference Gruszecki and Strzalka161). These mechanisms likely explain why inverse associations between carotenoid levels and disease risk have been observed for several age-associated conditions with an inflammatory or oxidative stress aetiology. Consequently, they may influence multi-system dysregulation which has been proposed to underlie the frailty syndrome.
Several studies have shown associations between lutein and zeaxanthin and frailty(Reference Michelon, Blaum and Semba62,Reference Semba, Bartali and Zhou63,Reference O'Halloran, Laird and Feeney68,Reference Rietman, Spijkerman and Wong162) , in addition to physical deficits including decreased grip, hip and knee strength in community-dwelling older women, according to a cross-sectional study(Reference Semba, Blaum and Guralnik163). Fig. 1 displays the results of a recent cross-sectional examination from our group that demonstrated that plasma lutein and zeaxanthin concentrations were negatively correlated with prefrailty and frailty across three different frailty instruments(Reference O'Halloran, Laird and Feeney68).
The role of lutein and zeaxanthin in cognitive impairment
Carotenoids have been proposed to have anti-inflammatory effects in addition to their antioxidant characteristics, by interacting with inflammatory cellular signalling cascades(Reference Ben-Dor, Steiner and Gheber159). Lutein and zeaxanthin – xanthophyll carotenoids with antioxidant and anti-inflammatory characteristics – are present in the retina and the brain and have neuroprotective properties. High concentrations of these carotenoids have been positively related to cognitive performance(Reference Feeney, O'Leary and Moran164). Higher plasma lutein and zeaxanthin were independently associated with better composite scores in the areas of executive function, memory and global cognition. Additionally, Feeney et al. discovered evidence linking increased plasma zeaxanthin with better processing speed(Reference Feeney, O'Leary and Moran164). Although the results of large population studies and clinical trials have been somewhat mixed, a recent review demonstrated a direct relationship among cognitive functions, macular pigment and the intake of lutein and zeaxanthin(Reference García-Romera, Silva-Viguera and López-Izquierdo165).
Dietary patterns, frailty and cognitive impairment
It is important to acknowledge the complex and synergistic relationships between nutrients. Vitamins and micronutrients often act as collections of co-factors, therefore interventions using singular or closely related compounds may have a focus that is too narrow. Interestingly, several studies have reported an increasing likelihood of frailty(Reference Michelon, Blaum and Semba62–Reference Bartali, Frongillo and Bandinelli64,Reference O'Halloran, Laird and Feeney68) with increasing accumulation of micronutrient insufficiencies. This is supported by a study that found supplementation with folate, vitamins B6, B12, D and calcium improved frailty among community-living older persons(Reference Ng, Feng and Nyunt166).
Because of the complex biological interactions between the various components of the diet, it has been suggested that using a whole-diet approach, through the study of dietary patterns rather than individual nutrients or food groups, might help to elucidate the role of diet in chronic diseases, such as frailty and cognitive impairment in older people. The Mediterranean diet is a good example of using dietary patterns to characterise dietary intake. Adherence to a Mediterranean-type diet pattern, known for its benefits on cardiovascular health and longevity(Reference Sofi, Macchi and Abbate167,Reference Psaltopoulou, Sergentanis and Panagiotakos168) , has also been linked to a decreased risk of frailty(Reference Bollwein, Diekmann and Kaiser169–Reference García-Esquinas, Rahi and Peres174) and cognitive impairment(Reference Yannakoulia, Kontogianni and Scarmeas175).
With respect to frailty, data from a 6-year longitudinal study revealed a lower risk of frailty in participants with a high Mediterranean diet score(Reference Veronese, Stubbs and Noale173). A recent meta-analysis, examining adherence to the Mediterranean diet and risk of frailty, indicated those with strongest adherence had a 56 % decreased risk of frailty(Reference Kojima, Avgerinou and Iliffe176). Further, a study found that older women with type-2 diabetes who were at risk for frailty from the nurses' health study benefited from better adherence to a Mediterranean diet(Reference Lopez-Garcia, Hagan and Fung177). Therefore, it seems in addition to consuming a Mediterranean-type diet to possibly treat frailty, later adoption of a Mediterranean-type diet may act as a limiting factor for the development of frailty(Reference Lopez-Garcia, Hagan and Fung177,Reference Bach-Faig, Berry and Lairon178) .
Other dietary patterns have shown comparable results. Higher healthy eating index scores were inversely related to lower risks of physical frailty in US older adults, according to Fan et al.(Reference Fan, Zhang and Li179). Another study using the ‘dietary inflammatory index’, a dietary pattern marker of foods and nutrient intakes related with inflammation, which may contribute to frailty, found that among 1948 participants who were tracked for up to 4 years, those with the highest adherence to the index had a higher risk of frailty and slower gait speed(Reference Laclaustra, Rodriguez-Artalejo and Guallar-Castillon180).
The majority of observational studies examining cognitive impairment point to an association between higher adherence to the Mediterranean diet and slower performance decline on various cognitive test batteries, as well as a decreased risk of dementia, mild cognitive impairment or progression from mild cognitive impairment to dementia. Two clinical trials that compared the Mediterranean diet pattern with nuts or olive oil to recommendations to limit dietary fat confirm these findings(Reference Valls-Pedret, Sala-Vila and Serra-Mir181,Reference Martínez-Lapiscina, Clavero and Toledo182) .
These approaches have the benefit of capturing potential interactions between microconstituents of diet, whether they are additive, antagonistic or synergistic(Reference Hu183). Applying similar approaches to the study of frailty and cognitive impairment may yield more informative insights than focusing on single nutrients alone.
Summary and way forward
In this review, we have focused on selected micronutrients that have been demonstrated to have a high prevalence of insufficiency and/or deficiency among older adults. We have reviewed the evidence, from TILDA and other studies, for their impact on age-related pre-frailty, frailty and cognitive decline. We have shown that low concentrations of folate and carotenoids are implicated in poorer cognitive health and that the co-occurrence of multiple nutrient deficiencies confers greatest risk for pre-frailty and frailty in the TILDA cohort of older adults. These findings are largely, if not consistently, supported by other epidemiological studies internationally. While the results from RCT often fail to support these relationships, there may be design reasons for this, such as relatively short follow-up times during RCT and the exclusion of those older adults with morbidities, frailty and cognitive problems. Inconsistent relationships for individual micronutrients with these outcomes in older adults may be overcome by assessing sup-optimal levels of several micronutrients simultaneously and using the accumulation of micronutrient insufficiencies/deficiencies, as demonstrated by TILDA data (Fig. 1). Single measurements can lead to misclassification, and the cut-off points routinely used to define deficiency may identify an acute rather than a chronic deficiency in older age groups. The complex synergistic interactions between nutrients are also important to consider. Vitamins and nutrients often function as a collection of co-factors, therefore interventions using singular or closely related compounds may have too narrow a focus. Also, given that older adults tend to experience malabsorption of nutrients, some micronutrients may be absorbed differently or less efficiently among older age groups. This may mean that definitions of micronutrient insufficiency and deficiency may be less accurate at older ages and chronic low/sub-optimal status could have less well-understood negative impacts on health. These biological changes coupled with diminution of appetite may partly explain the consistent observation that older adults struggle to maintain sufficient dietary intakes and circulating levels of several micronutrients with advancing age. Among previous TILDA studies, our group has demonstrated that the current custom of voluntary micronutrient fortification in Ireland is not effective in maintenance of sufficient micronutrient status among older age groups(Reference Laird, O'Halloran and Carey96,Reference Laird, O'Halloran and Carey122) .
Sustaining the health of a globally ageing population requires strategies that will prolong healthspan by delaying the onset of age-related diseases until later in the life course. Therefore, it is important to focus on modifiable factors, such as micronutrients, that can be intervened upon, particularly in ‘at-risk’ groups if they can be identified early, i.e. those who have pre-frailty and/or indications of cognitive decline. Testing for micronutrient insufficiencies/deficiencies in these ‘at-risk’ groups may be used to both monitor the health of older adults and as an intervention target to support biological function and decelerate biological ageing and the onset of physical and cognitive decline. To achieve this, public health policies and awareness programmes are required that highlight the importance of maintaining micronutrient sufficiency via mandatory fortification and/or supplementation to support health as we age.
Acknowledgements
The authors acknowledge the contribution of the participants in the study, members of the TILDA research team, study nurses and administrative team.
Financial Support
Original funding for TILDA was provided by The Atlantic Philanthropies, the Irish Government and Irish Life plc. The sponsors played no role in designing or conducting the study or in the collection, management, analysis or interpretation of the data, nor did they have any input into the preparation, review or approval this paper.
Conflict of Interest
None.
Authorship
The authors had sole responsibility for all aspects of preparation of this paper.






