- AAF
amino acid-based formulas
- eHF
extensively-hydrolysed formulas
- FHS
food hypersensitivity
In 2004 the European Academy for Allergy and Clinical Immunology and WHO published the revised nomenclature for food hypersensitivity (FHS) as guidance for the management of allergic diseases(Reference Johansson, Bieber and Dahl1). This guidance publication identifies FHS as the umbrella term for both immune- and non-immune-mediated reactions to food. Food allergy is distinguished from other adverse reactions by an immune-mediated mechanism, whereas food intolerances do not involve the immune system. If the allergic response involves serum IgE, it is classified as an IgE-mediated food allergy, which usually occurs within 2 h of allergen consumption(Reference Atkins2). A non-IgE-mediated food allergic response is thought to be a T-cell-mediated reaction and is often referred to as a delayed food allergy(Reference Ferreira and Seidman3) (Fig. 1).

Fig. 1. Proposed nomenclature for food hypersensitivity(Reference Johansson, Bieber and Dahl1).
Symptomatology varies according to the type of hypersensitivity reaction (Table 1), and determining the type of reaction, i.e. immunological mechanisms involved(Reference Atkins2), assists in the diagnostic work-up, management strategies and the prognosis of the FHS. For example, cow's milk-protein allergy may present with IgE-mediated symptoms such as urticaria or angioedema or non-IgE-mediated gastrointestinal symptoms such as protracted diarrhoea and marked abdominal discomfort(Reference Kvenshagen, Halvorsen and Jacobsen4). Treatment and prognosis of these two immune responses to cow's milk differ.
Table 1. Clinical presentation of food hypersensitivity (adapted from Venter(Reference Venter, Skypala and Venter28))
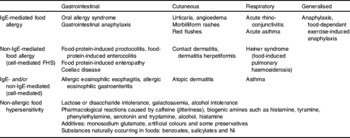
FHS usually manifests in early childhood and is mainly caused by eight foods: cow's milk; hen's egg; soyabean; peanuts (Arachis hypogaea); tree nuts; wheat; fish; shellfish. The prevalence of FHS in 0–3 year olds ranges between 2·1% and 4·2%(Reference Venter, Pereira and Voigt5–Reference Roehr, Edenharter and Reimann7). The reported prevalence of true food allergies in adults varies between 1·8% and 4%(Reference Osterballe, Hansen and Mortz6, Reference Osterballe, Hansen and Mortz8–Reference Zuberbier, Edenharter and Worm12). The foods implicated in adulthood include wheat, cow's milk, egg, soyabean, citrus, fish and shellfish, pork, alcohol, menthol, additives and glucose, chocolate and cocoa, fruit and vegetables and peanuts and tree nuts.
The annual fiscal burden of allergic disease in the UK is estimated to be £900×106(13), mostly through prescribed treatments in primary care, which represents 10% of the general practitioners prescribing budget. The diagnosis of FHS is facilitated by a medical history, blood tests, skin prick test or a targeted elimination diet(Reference Atkins2). The use of blood tests and skin prick tests are helpful in the diagnosis of IgE-mediated food allergy and require careful interpretation. The best method for diagnosing IgE-mediated allergy and the only method for non-IgE-mediated allergy and food intolerances (non-allergic FHS) is an elimination diet followed by re-introduction of the relevant food or a food challenge. Dietetic expertise is of particular importance in advising and monitoring of elimination diets(Reference Venter, Vlieg-Boerstra, Carling, Skypala and Venter14). The use of elimination diets for the diagnosis of FHS has previously been discussed(Reference Grimshaw15).
Dietary management of food hypersensitivity
The main goals in the management of FHS are to prevent the occurrence of acute and chronic symptoms. In children it is important to maintain optimal nutrition for growth and development(Reference Vlieg-Boerstra, van der Heide and Bijleveld16). Conversely, for adults the emphasis is on preventing micronutrient deficiencies and excessive weight loss(Reference Kershaw, Skypala and Venter17). These management objectives require regular dietary assessment and monitoring. Whilst the mainstay of treatment is allergen avoidance, more severe reactions may require the use of antihistamines and/or adrenaline. Other novel treatment and management modalities include oral and sublingual immunotherapy(Reference Skripak and Sampson18), anti-IgE treatments(Reference Skripak and Sampson18), Chinese herbal medication(Reference Skripak and Sampson18) and the use of pre- and probiotics(Reference Wallace19, Reference Veereman-Wauters20); however, their routine use requires further research. The present review will focus only on the dietary management of FHS and its challenges.
The EU considers cereals containing wheat and gluten, shellfish, eggs, fish, peanuts and tree nuts, cow's milk, celery, mustard, sesame seeds (Sesamum indicum), molluscs, soyabean, lupin (Lupinus spp.) and SO2 as the most common food allergens(21). Table 2 shows information on the major food allergens.
Table 2. The fourteen major food allergens according to the EU: their sources, terminology used, nutrients, alternatives, mechanisms involved and level of avoidance required

eFH, extensively-hydrolysed formula; AAF, amino acid-based formula; FHS, food hypersensitivity.
* Almonds, Amygdalus communis L.; hazelnuts, Corylus avellana; walnuts, Juglans regia; cashews, Anacardium occidentale; pecan nuts, Carya illinoiesis Wangenh. K. Koch; Brazil nuts, Bertholletia excelsa; pistachio nuts Pistacia vera; Queensland nuts, Macadamia ternifolia.
† Rice milk is not recommended in the UK for children <4·5 years of age(101).
‡ One to two teaspoons fruit puree for binding; 1·5 teaspoons water, 1·5 teaspoons oil, one teaspoon baking powder; one teaspoon baking, powder, one teaspoon liquid, one teaspoon vinegar; one packet gelatine, two teaspoons warm water; one teaspoon yeast dissolved in 0·25 cup warm water(Reference Wright, Meyer, Skypala and Venter58).
§ Sources of lactose in pharmaceutical preparations must be avoided if total exclusion is required, which may not apply to the majority of cases; butter and hard cheeses can usually be included because their lactose content is either very low or they do not contain lactose at all. In a recent UK study researchers have found undetectable quantities of lactose in Gruyere, Emmental, Jarlsberg, Parmigiano Reggiano and Grana Padano Italian Parmesan and mature Cheddar cheese from the UK West Country Farmhouse Cheese Makers Association only and lactose in other mature Cheddar cheeses, Gouda and Edam(Reference Portnoi and Macdonald103). Yoghurt and other fermented-milk products may also be tolerated by some individuals who are able to tolerate small amounts of lactose. Commercially-available lactose-reduced milks may be useful for individuals with temporary or partial lactose intolerance; however, they are not suitable for individuals with congenital alactasia or infants <12 months of age.
Many of these foods contribute substantially to dietary adequacy in patients and pose major nutritional challenges in the management of allergic disease. These challenges include: (1) determining the level of food avoidance required; (2) appropriate avoidance of the food; (3) ensuring adequate nutritional intake; (4) assessing and monitoring nutritional status; (5) determining development of tolerance.
Challenge 1: determining the level of avoidance required
The mainstay of treating FHS is the avoidance of the relevant food(s) from the individual's diet. However, a common dilemma in clinical practice is the extent of dietary avoidance. Complete avoidance, including traces of the allergen, is difficult to follow and has a major impact on quality of life(Reference Avery, King and Knight22, Reference Sicherer, Noone and Munoz-Furlong23). This advice may not be essential for those who already tolerate small amounts of the relevant foods; strict avoidance in these patients may lead to serious reactions when accidentally ingested(Reference Flinterman, Knulst and Meijer24).
Levels of avoidance required are currently based on: type of FHS involved; characteristics of the particular food protein; natural history of the particular FHS; the nutritional status of the patient.
Type of food hypersensitivity involved
This level of avoidance relates to the immunological mechanisms involved. Most individuals with IgE-mediated food allergy need to completely avoid the food and even trace amounts of the food. However, some patients are able to tolerate small amounts of the allergen, e.g. extensively-heated egg or milk (i.e. biscuits, cakes), despite reacting to other forms of these foods such as raw egg or pasteurised milk(Reference Lemon-Mule, Sampson and Sicherer25, Reference Nowak-Wegrzyn, Bloom and Sicherer26). The decision to allow small amounts in food should be made on an individual basis together with an allergist.
In the majority of severe cases of non-IgE-mediated allergy such as severe eczema, food-protein-induced enteropathies and eosininophilic disease complete avoidance of the allergen will be required(Reference Hill, Murch and Rafferty27). However, some individuals with non-IgE-mediated food allergy (i.e. moderate gastrointestinal presentations) may be able to tolerate small amounts of the food to which they are allergic. Tolerance of trace amounts in non-IgE-mediated food allergies has not been defined by published research and should also be managed on an individual basis.
Most individuals with non-allergic food hypersensitivity will be able to include small amounts of the food or substance in their diet with no adverse effects. In these individuals the adverse reactions depend not only on the presence of the food, but also the amount ingested. For example, reactions to histamine-containing foods could be caused by several factors such as: amount of histamine produced and released intrinsically; histamine production by gut bacteria; dietary intake of foods containing or releasing histamine; catabolic enzymes not able to reduce excess histamine within the body(Reference Venter, Skypala and Venter28).
Characteristics of the particular food protein
Individuals with nut allergies are advised to completely avoid all nuts in any form(Reference Towell, Skypala and Venter29), whereas some individuals with egg allergy may be able to tolerate small amounts of well-cooked egg(Reference Lemon-Mule, Sampson and Sicherer25).
Natural history of the particular food hypersensitivity
Most children will outgrow their milk allergy at some point during childhood(Reference Venter, Pereira and Voigt5, Reference Host, Jacobsen and Halken30, Reference Skripak, Matsui and Mudd31), but only about 20%(Reference Fleischer, Conover-Walker and Christie32) will outgrow their nut allergy.
Nutritional status of the patient
Unnecessary avoidance of food allergens can further impair nutritional status.
Challenge 2: appropriate avoidance of the food(s)
Avoidance advice
Healthcare professionals should give patients and/or carers clear guidance about food avoidance to prevent both unnecessary restrictions and accidental exposure to allergens. Table 2 provides a checklist of foods and ingredients to avoid when suffering from food hypersensitivity.
Preventing cross-contamination
In order to prevent cross-contamination individuals should be advised to wash cooking utensils thoroughly, take special care when washing chopping boards or work surfaces and wash hands thoroughly(Reference Simonte, Ma and Mofidi33); also, they should not use the same oil for cooking different foods and not to use the same spoon for serving different food(Reference Wright, Skypala and Venter34).
Eating away from home
Eating away from home can be problematic as individuals will not always have reliable ingredient information to assess allergen exposure. It is crucial to ask questions about ingredients and food preparation or possible cross-contamination. If possible, individuals should call ahead and ask to speak with the individual who prepares the food (e.g. chef when going to a restaurant) who can give information about the menu and discuss alternative safe options.
It is quite common for individuals with allergies to carry a ‘chef card’(35, 36) that outlines the foods that require avoidance; when eating in a restaurant such cards can be very useful. They are available from a variety of distributors across Europe.
The nutritional burden of food avoidance can be important in the growing child(Reference Ojuawo, Lindley and Milla37, Reference Bhaskaram38). It is essential that suitable alternatives or supplements are provided to ensure normal growth. Carers of children with food allergies also benefit from practical advice (i.e. suitable snacks, birthday cakes) for nursery, for school and for children's birthday parties. Carers should also be advised on how to minimise the effect of ostracising the child and how to use emergency medication if prescribed(Reference Wright, Skypala and Venter34). The dangers of children exchanging food items provided in lunch boxes should also be discussed and clarity should be provided on the exact meaning of specific ‘food bans’ at the playgroup or school. This approach will be similar for dealing with away-days and school trips.
For adults, managing FHS at work can be challenging. It is important to take suitable food to work to minimise the risk of becoming hungry and having to check foods and food labels at work. Special occasions at work should be dealt with in a similar way to eating away from home.
Going on holiday
Holidays should be an enjoyable occasion, but for those individuals needing to adhere to strict avoidance measures some planning is required. Whether holidays are in the country of origin or abroad, self-catering is in most cases the best option. Some hotels are happy to discuss dietary restrictions and may accommodate nutritional requirements.
For those going on holiday abroad it is advisable to obtain the following information beforehand: ingredients of foods served on the flight, train or boat; where the nearest emergency unit is and the contact details; translation cards can be obtained from a number of sources such as Allergy Action(39) or Allergy UK (British Allergy Foundation)(40). These translation cards provide information on the patient's FHS in the local language in addition to food ingredients to look out for when travelling.
The Anaphylaxis Campaign (see Table 3) is also able to provide useful information to individuals with food allergies.
Table 3. Useful websites for healthcare professionals managing food hypersensitivity

Finally, prescribed medication should be carried and a medical alert armband or necklace worn at all times(Reference Wright, Skypala and Venter34). A letter from a healthcare professional is useful for airlines when carrying these individuals onboard.
Understanding food labels
Food labelling legislation differs across the world and healthcare providers should obtain information relating to domestic food labelling laws, in particular the labelling of food allergens. Individuals should be shown how to read food labels to identify relevant ingredients.
In the EU the food labelling law for pre-packed foods effective from 25 November 2005 was updated in 2007(21). This legislation requires that all pre-packed food, including alcoholic drinks, sold in the UK or the rest of the EU clearly list all ingredients, including any of the major allergens, even if only present in small amounts.
The ‘25% rule’ has been abolished under the new legislation in which individual components of a compound ingredient making up <25% of the finished product do not have to be listed. However, the EU directive does not abolish ‘may contain’ statements on labels. Thus, even though an allergen (particularly used for nuts, seeds and milk) is not deliberately included in the food, the manufacturer cannot ensure that the product does not contain traces as a result of, for example, manufacturing other products within the same factory. The Food Standards Agency advises that these statements (advisory labelling) should only be used following a thorough risk assessment and if it is considered that there is a real risk of allergen cross-contamination(41). Despite this guidance, it has been reported that many families consider the widespread use of ‘allergen traces’ labelling on pre-packed foods (particularly those aimed at or widely consumed by children) and everyday staples (bread, cereals and ordinary biscuits) to be the major obstacle to leading a normal life(Reference Gowland42, 43).
A further important point to note about labelling is that allergen statements such as milk-free, egg-free etc. are not compulsory to give, although it is given by some manufacturers(41). It is therefore prudent not to assume that if there is no allergy statement present on the label that a product is free from the allergenic foods. Healthcare professionals should always advise patients to read and take note of the ingredient list and not to rely on the information box, as this source may not give all the information.
Lifestyle issues arising when avoiding certain foods
Research has indicated that 39% more time is spent on shopping in families with a member who has a food allergy(Reference Primeau, Kagan and Joseph44). More importantly, a study has indicated that the quality of life of children with peanut allergy is worse than that of children living with diabetes(Reference Avery, King and Knight22). Living with dietary restrictions therefore requires substantial changes in lifestyle in order to ensure the appropriate level of avoidance, particularly if complete avoidance of the food(s) is needed. Healthcare professionals can help to minimise the effect on quality of life for both the patient and the family with the provision of information (as discussed earlier).
What can be learned from fatalities?
A report has indicated that only half the individuals who are known to have died from food allergy in the UK had been actually trying to avoid the food implied in their death(Reference Pumphrey and Gowland45). Foods that have been reported to be associated with fatalities include milk, peanuts, nuts, fish, shellfish, snail, sesame, egg and tomato. It has not been possible to identify the foods in a number of cases. Most of the fatal reactions were found to have happened at home or at a friend's or relative's house, followed by work, school, nursery, in a restaurant, out and about, at camp and at a wedding reception. The food blamed for fatal reactions was reported to be catered, prepared at home, in a package and labelled, sold loose or unlabelled, whole nuts and in three cases unknown.
On the other hand, all fatalities in the USA since 2001 have been reported to have been caused by known allergens(Reference Bock, Munoz-Furlong and Sampson46). These reactions were reported to have happened at home, a friend's house, at school, restaurants and buffets or when camping. In some cases it was found that the individual did not ask for the ingredients, but in some cases they did ask and were wrongly informed. It was found that the foods involved in the fatalities involved a wide range of foods such as cakes, biscuits, sweets, sauces, ethnic foods and nut mixes or were caused by cross-contamination. Most fatality cases had presented only with mild symptoms in the past.
The most important messages from these two reviews are to always ask about ingredients, to insist that the ingredients are checked, to always carry emergency treatment and to treat any reaction immediately whenever possible.
Challenge 3: ensuring adequate nutritional intake
The nutritional implications of any avoidance diet will depend on a number of factors(Reference Kershaw, Skypala and Venter17, Reference Thomas and Bishop47).
Frequency of normal consumption of the food(s) and avoidance of particular food group
Avoidance of a single food or type of food that is not eaten regularly (such as kiwi fruit (Actinidia deliciosa)) will be of little importance. In contrast, avoidance of a single food or food group that is considered to be a staple food and contributes substantially to nutritional adequacy (such as wheat in adults or cow's milk in children) will be of considerable importance (Table 2).
Frequency of ready-prepared food consumption
In the UK the tendency to cook at home from fresh ingredients has reduced, resulting in an increase in the reliance on ready-made meals and takeaways. This change, which is mainly related to convenience and cost(Reference Pettinger, Holdsworth and Gerber48), markedly complicates the exclusion of allergens, which often are present in only small amounts (i.e. nuts and soyabean) in manufactured foods. Wheat allergy poses a particular problem, as it is not only considered to be a staple food but is also used in a large number of commercial products such as bread, cereals, cakes and biscuits and pasta(Reference Mofidi49).
Additional food avoidance not related to food hypersensitivity
Patients requiring special attention are those who exclude foods for cultural, religious (e.g. kosher foods) or ethical reasons and those with particular food preferences in addition to their FHS. For example, a vegan who is allergic to nuts will need considerably more nutritional input to assure dietary adequacy.
The number of allergens avoided
The more foods that require to be avoided, the more the nutritional quality of the diet is affected(Reference Grimshaw15). This position is related to the limited availability of nutritionally-suitable alternatives, with a consequent adverse effect on dietary adequacy. Avoidance of a large number of foods increases the likelihood of the individual losing their interest in food, which may have an additional impact on food intake, particularly in children(Reference Kershaw, Skypala and Venter17).
Period of elimination
The nutritional impact of a short-term exclusion diet (4–6 weeks) is likely to be minimal. However, if the exclusion diet is likely to last for a longer period, i.e. for life, the impact on a patient's nutritional status could more be important(Reference Kershaw, Skypala and Venter17).
Nutrient content of major food allergens and how to ensure adequate intake
In order to ensure a nutritionally-adequate diet whilst avoiding some foods from their diets, individuals need the following information(Reference Kershaw, Skypala and Venter17): most important nutrients in the food or food group that are being avoided (Table 2); a list of alternative foods high in a particular nutrient(s) to substitute nutrients that are omitted from the diet as a result of the FHS; nutritional supplements, especially in the infant and growing child with an allergy(Reference Ojuawo, Lindley and Milla37, Reference Noimark and Cox50).
Further points that need to be taken into account to ensure nutritional adequacy include: advice on suitable foods; special considerations for children with cow's milk-protein allergy.
Advice on suitable foods
There are a number of ways to identify suitable foods for an individual with an allergy(Reference Wright, Skypala and Venter34). Some retailers and manufacturers provide ‘free from lists’ for their own brands. This information is very useful and can often be obtained from the retailer or manufacturer. Although specifically-manufactured food products are available for patients with allergies, many everyday foods are suitable for individuals with FHS. Healthcare professionals, in particular dietitians, can assist patients to modify recipes suitable for their FHS or obtain an ‘allergy-free’ recipe book with trialled recipes. However, many family recipes will still be suitable for a restricted diet, possibly with minor adaptations. Table 3 summarises useful websites for healthcare professionals managing FHS.
Special considerations for children with cow's milk-protein allergy
The management of cow's milk allergy requires advice for breast-feeding mothers and/or assistance in choosing an appropriate hypoallergenic formula where mothers' choose not to breast-feed or need a supplement to breast milk(Reference Host, Koletzko and Dreborg51).
Mothers who breast-feed should be encouraged to continue and a maternal cow's milk-exclusion diet is therefore the first line of treatment. If the maternal elimination diet does not lead to any symptom improvement, a normal diet should be resumed or other allergies need to be considered. Special attention should be given to infants with atopic dermatitis, proctitis and enterocolitis(Reference Hill, Murch and Rafferty27), as components other than bovine β-lactoglobulin or casein have been shown to induce allergic symptoms in children who are exclusively breast-fed and may therefore continue to exhibit allergic symptoms(Reference Restani, Gaiaschi and Plebani52). In some cases an amino acid-based formula (AAF) may be indicated despite careful avoidance of cow's milk or other relevant foods. This decision to stop breast-feeding should not be taken lightly as it is difficult to reverse. A discussion with the mother and an appropriately-qualified healthcare professional is indicated.
Formulas that are suitable for the management of cow's milk allergy are extensively-hydrolysed formulas (eFH) and AAF. The European Society of Paediatric Gastroenterology, Hepatology and Nutrition and the European Academy of Allergy and Clinical Immunology stipulate that ‘dietary products for treatment of cow's milk protein allergy in infants should be tolerated by at least 90% (with 95% confidence) of infants with documented cow's milk protein allergy’(Reference Host and Halken53).
A number of studies have evaluated the use of eHF based on casein and whey and AAF for the management of cow's milk allergy. A systematic review has indicated that eHF and AAF are equally effective in relieving the symptoms of cow's milk allergy in young children(Reference Hill, Murch and Rafferty27). However, infants suffering from non-IgE-mediated food-induced gastro-enterocolitisproctitis syndromes with failure to thrive, severe eczema and severe reflux oesophagitis or with symptoms during exclusive breast-feeding are more likely to benefit from AAF than eHF. Infants on AAF also seem to have better longitudinal growth than infants on eHF(Reference de Boissieu, Matarazzo and Dupont54, Reference de Boissieu, Matarazzo and Rocchiccioli55). It has also been recommended that infants presenting with severe manifestations of cow's milk-protein allergy such as failure to thrive, hypoproteinaemia and hypoalbuminaemia or Fe-deficiency anaemia should start with AAF(Reference Vandenplas, Koletzko and Isolauri56). Similarly, infants and children with multiple food allergies often have more severe features, with possible reactions even to small quantities of antigens, and are thus unresponsive to eFH and have a late acquisition of tolerance(Reference Hill, Hosking and Heine57).
It is important to take the cost of different treatment options for cow's milk allergy into account against the cost of hospitalisation, investigation and increased morbidity in infants for whom effective treatment is delayed. Further health economic data are required to assist healthcare workers in this context(Reference Hill, Murch and Rafferty27).
A major problem with the use of hypoallergenic formulas is their poor palatability(Reference Wright, Meyer, Skypala and Venter58). However, infants <6 months of age have a relatively ‘naïve’ taste perception and therefore usually accept these formulas without difficulties. Older infants and those who have been previously breast-fed commonly reject the introduction of hypoallergenic formula(Reference Menella, Forestell and Morgan59).
Thus, the hypoallergenic formula should be introduced as soon as possible or if the infant is being breast-fed continue to do so until 1 year of age when other more-palatable alternatives may be considered. Depending on the severity of the symptoms, transitional introduction may be considered with incremental mixing of the milks. The hypoallergenic formula can be offered as the main fluid source. If the infant is >6 months introduce the formula in a feeder beaker that has good flow (avoid beakers with valves). The smell can be masked with a good-quality vanilla essence (a few drops only; it should be noted that vanilla essence does contain alcohol and excessive addition is not indicated and can also make the formula bitter). Commercial milk-free milkshake powders should be used as a last option as they could create preference for a ‘sweet taste’ if used. Their concentration should be reduced over time until the formula is taken neat.
In the UK soya formulas are not recommended for infants aged <6 months(60). Although it is also not recommended as the first choice of formula for infants aged between 6 months and 12 months who are allergic to cow's milk, it can be useful for infants not allergic to soyabean who refuse hypoallergenic formulas(61, Reference Guest62). The prevalence of concomitant soyabean allergy in infants with cow's milk allergy differs between IgE- and non-IgE-mediated disease(Reference Agostoni, Fiocchi and Riva63). It ranges between 10% and 14% in infants with IgE-mediated allergy(Reference Zeiger, Sampson and Bock64, Reference Klemola, Kalimo and Poussa65), but in non-IgE-mediated cow's milk allergy it is markedly higher, especially in enterocolitis and enteropathy syndromes(Reference Agostoni, Fiocchi and Riva63).
For children >2 years of age with a nutritionally-complete diet and good growth cow's milk alternatives include Ca-enriched soya, almond (Amygdalus communis L.), pea (Pisum sativum), oat, coconut (Cocos nucifera) and potato milks or the more rare alternatives such as quinoa (Chenopodium quinoa) drink and chufa milk made from tigernuts (Cyperus esculentus).
A number of factors play a role in the decision to move children from an infant formula to commercially-available milk substitutes including: the volume of formula that is consumed and amount of solid food eaten, e.g. a child with feeding issues who is still taking a substantial volume of formula may be left on the formula for much longer or a formula for older children may be chosen; the alternatives to cow's milk available locally and the nutritional profile of the milk substitute (Table 4); growth profile; energy requirements v. energy intake(Reference Groetch66).
Table 4. Comparison of the different milks (/100 ml)Footnote *
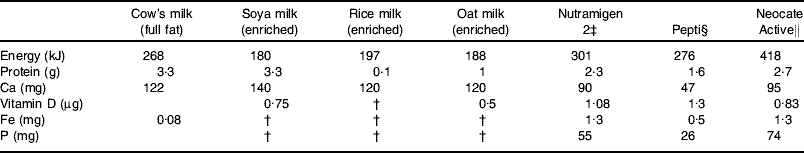
* Values for cow's milk and soya milk are taken from Food Standards Agency(104) and values for all other milks are derived from information provided by manufacturers.
† Negligible amounts present.
‡ Mead Johnson Nutrition, Uxbridge, Middlesex, UK; extensively-hydrolysed casein formula, suitable for infants >6 months of age.
§ Nutricia Ltd, Trowbridge, Wilts., UK; extensively-hydrolysed whey formula, suitable from birth.
|| Nutricia Ltd; amino acid-based formula, suitable from 1 year of age.
Food refusal is commonly seen during infancy. It is thought that 16·7% and 18·8% of 8-month-old and 12-month-old infants respectively have severe aversive feeding behaviour(Reference Wright, Parkinson and Drewett67). Research indicates that infants with reflux-type symptoms or colic, which are often related to non-IgE-mediated allergy to cow's milk and soyabean, are associated with major feeding problems that range from food refusal, gagging on introduction of lumpier textures and extreme anxiety during meal times(Reference Mathisen, Worrall and Masel68, Reference Miller-Loncar, Bigsby and High69). Studies on the prevalence of feeding difficulties in children with IgE-mediated food allergies have not been conducted. Children with food allergies and concomitant feeding difficulties pose a major nutritional challenge, as food choices are often further limited because of important sensory hypersensitivity symptoms: texture aversion; colour specificity; temperature; smell; taste specificity. These feeding problems should ideally be managed within a multidisciplinary team and should address the following issues: ensure that the child does not have undiagnosed allergies leading to continuing discomfort; nutritional adequacy of the diet; meal-time routine and feeding times; sensory hypersensitivity; behavioural management.
Challenge 4: assessing and monitoring nutritional status
Initial assessment
A nutritional assessment can provide useful information that can be used as a baseline for monitoring the nutritional status and the impact of the avoidance diet(Reference Kershaw, Skypala and Venter17). More importantly, this information may affect the management (avoidance) strategy that will be implemented. For example, a young child with faltering growth related to multiple food allergies will require avoidance advice as well as advice on how to increase energy, protein and vitamin and mineral intake. If an adult with a history of an eating disorder is seen about irritable bowel syndrome less stringent advice may be given than when the same patient is seen with a history of anaphylaxis caused by peanut ingestion. It is therefore important to weigh up the nutritional implications of the diet against the severity and extent of the symptoms.
Monitoring nutritional status
Monitoring height and weight
When dealing with children the simplest way of monitoring for nutritional deficiencies is to assess growth velocity using the nationally-recognised growth curves(70). Measuring the growth of infants, toddlers and children can help to detect growth-related concerns: excessive weight gain; weight faltering; wasting and stunting. It can also provide reassurance about normality(71).
In an adult population monitoring weight, height, BMI or other anthropometric measurements can be used to assess nutritional status(Reference Thomas and Bishop72, Reference Thomas and Bishop73).
Monitoring dietary intake
It is known that an individual can have a poor nutritional status despite having a normal BMI or even being overweight(Reference Mofidi49, Reference Thomas and Bishop72). For individuals with FHS it is particularly important to assess the intake of micronutrients as well as that of macronutrients. A variety of dietary intake measures may be used, e.g. 24 h recall and 1–7 d food diaries, each of which have their own limitations(Reference Burley, Cade and Margetts74). Intakes may be analysed and compared against the national recommended nutrient intakes (for UK recommended nutrient intakes, see Department of Health(75)).
It has been suggested that for infants >6 months a 24 h recall may be used(Reference Mofidi49). However, a 3 d diet record is necessary for a child aged ≥6 months and for adults because day-to-day intake is more varied. The interpretation of dietary intake data requires a qualified dietitian. In some cases it may also be necessary to consider the assessment of biochemical markers(Reference Mofidi49). The combination of a dietary assessment and assessment of biochemical markers can aid the recommendation to supplement the intake of a particular vitamin and/or mineral.
Adverse outcomes of exclusion diets
The nutritional contribution of any allergenic foods that are being excluded and the number of foods excluded from the diet must be carefully considered(75, Reference Feeney76). A number of reports have documented inadequate energy intake that leads to growth faltering in children(Reference Noimark and Cox50, Reference Christie, Hine and Parker77–Reference Arvola and Holmberg-Marttila81). In particular, insufficient intake of vitamin D presenting as rickets(Reference Noimark and Cox50, Reference Fox, Du and Lang82), hypocalcaemia(Reference Noimark and Cox50), Fe-deficiency anaemia(Reference Noimark and Cox50), essential fatty acid deficiency(Reference Aldamiz-Echevarria, Bilbao and Andrade83) and kwashiorkor in conjunction with multiple nutrient deficiencies(Reference Liu, Howard and Mancini84, Reference Carvalho, Kenney and Carrington85) have been reported.
Three studies have addressed growth in children with multiple food allergies(Reference Christie, Hine and Parker77, Reference Isolauri, Sutas and Salo86, Reference Niggemann, Binder and Dupont87). A study of 100 infants (<12 months of age) with cow's milk-protein allergy has found that their length and weight-for-length indices are decreased compared with those of age-matched healthy controls(Reference Isolauri, Sutas and Salo86). Furthermore, age at the onset of symptoms and duration of the elimination diet are major contributors to growth impairment. An evaluation of the efficacy of AAF compared with eHF in children with cow's milk allergy has found improved growth (length) in those taking AAF despite similar energy intakes(Reference Niggemann, Binder and Dupont87). This finding has been confirmed by work showing that children with two or more food allergies are shorter, based on height-for-age percentiles, than those with one food allergy and their intakes of Ca, vitamin D and vitamin E are also lower than the recommended daily intakes(Reference Christie, Hine and Parker77).
These studies have identified maternal fears(Reference Noimark and Cox50), no dietetic referral(Reference Liu, Howard and Mancini84, Reference Niggemann, Binder and Dupont87), alternative allergy testing(Reference Noimark and Cox50), use of an inappropriate alternative formula(Reference Carvalho, Kenney and Carrington85) or palatability of the chosen formula(Reference Noimark and Cox50) as important indicators of a poor nutritional status. This evidence reinforces the need for all food-avoidance diets to be supervised by an appropriate specialist healthcare professional with nutritional knowledge such as a registered dietitian(Reference Liu, Howard and Mancini84, Reference Niggemann and Heine88).
Challenge 5: determining development of tolerance
Patients should be re-assessed frequently in order to determine development of tolerance to a food. There are a number of factors that play a role in determining the tolerance to food(s). The regular assessment of nutritional status during a period of food avoidance will assist in the decision as to whether to continue an elimination diet or consider food challenges in an attempt to broaden the scope of an individual's diet.
Cow's milk and egg allergy is often transitory and mostly is resolved by 5 years of age(Reference Venter, Pereira and Voigt5). The timing of reintroduction will vary according to the clinical circumstances. If previous reactions have been severe IgE-mediated reactions or other food allergies have developed reintroduction may be delayed for longer. The decision to reintroduce food allergens should always be taken by an appropriately-qualified clinician and may require supervision in hospital. It is now also known that there is a possible risk of enhanced reaction after a period of withdrawal(Reference Flinterman, Knulst and Meijer24); however, this outcome is specific to children with a history of delayed symptoms only, for whom a risk of developing immediate more-severe symptoms during a ‘home challenge’ does exist. It is therefore best clinical practice to repeat skin-prick testing or specific IgE levels before to a food challenge. When the size of the skin-prick test or specific IgE levels are the same or even greater the challenge should be postponed; this provision applies to all foods. In the case of cow's milk, persistence into childhood and adulthood necessitates permanent exclusion. It is often the case that following a negative challenge the child does not want to reintroduce the food into the diet(Reference Eigenmann, Caubet and Zamora89) because of anxiety or dislike of the taste, and parents will need guidance on ways of incorporating these foods into the diet.
Very little is known about development of tolerance to either foods or ingredients in adults.
Conclusion
Avoidance of the relevant food is the mainstay of the management of FHS. Before any dietary avoidance is implemented the individual should be medically assessed and diagnosed appropriately. In some cases an elimination diet may be needed for diagnostic purposes. There is no validated tool or guide available for the management of FHS. In order to ensure appropriate avoidance of the food whilst the individual maintains a good nutritional status a dietary consultation should include: (1) assessment of height, weight and dietary intake; (2) avoidance advice including understanding food labels and lifestyle issues; (3) information on substitute foods, ‘free from’ lists and special dietary products; (4) follow-up and reassessment to determine development of tolerance.
Acknowledgements
The authors declare no conflict of interest. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. C. V. prepared a skeleton outline of the paper. C. V. and R. M. then prepared their allocated sections and edited each other's contributions.







