It is thought that meat was incorporated into the diet of our ancestors at least 2·6 million years ago( Reference Pobiner 1 ). Inclusion of meat into the diet played a key evolutionary role in man. It has been suggested that without the inclusion of animal source foods in the diet, it is unlikely that evolving man could have achieved their unusually large and complex brain while simultaneously continuing their evolutionary trajectory as large, active and highly social primates( Reference Milton 2 ). Red meat continues to play an important role in the human diet by providing a good source of high-quality protein as well as beneficial fatty acids and a variety of micronutrients for optimal health.
Red meat is commonly considered to include beef, pork, lamb and game. Processed meat is generally defined as any meat preserved by salting, smoking, curing, or by the addition of chemical preservatives, such as bacon, sausages, salami or ham( Reference Larsson and Orsini 3 ). In recent years, red meat has attracted much debate regarding its impact on health and the environment. Consumption trends of meat vary greatly around the world. Significant increases in consumption are apparent in developing countries with Latin America, the Caribbean and East Asia seeing particularly large increases( 4 ). The latest data from the UK Family Food Survey (2013)( 5 ) reported a decrease in the quantity of household purchases of carcass meat (beef, veal, mutton, lamb and pork) from 211 g in 2010 to 182 g in 2013. Data from the National Diet and Nutrition Survey indicate that diets in the UK can be low in nutrients typically found in red meat. Some population groups, in particular, have low intakes of important micronutrients and red meat can play a significant role in helping such groups meet nutritional requirements. In the present paper, red meat's nutrient contribution to the UK population is explored and in particular its role in providing key nutrients in the diet of young infants, adolescents, women of child-bearing age and older adults. The impact of red meat on weight loss and weight maintenance is discussed as well as how much red meat should we eat.
Nutrient contribution of red meat to UK population
Macronutrients
Red meat contains high biological value protein with all eight essential amino acids required by adults and all nine required by children. Protein is needed for growth, maintenance and repair of the body. Red meat contains on average 20–24 g protein per 100 g (when raw) and can therefore be considered a high source of protein. In most developed countries, average protein intakes are above the minimum protein requirements for good health. Any excess protein in the diet is used to provide energy. The amount of energy provided by red meat is variable. Fat provides the richest dietary source of energy and wide variation of fat content can be seen in red meat, depending on the type, the cut and degree of trimming( Reference Higgs 6 ). The type of fat, as well as the total fat content is important to consider in terms of CVD, as not all fats are equal. Different fatty acids have different effects on blood cholesterol and risk of heart disease, some beneficial and some adverse. The fatty acid profile of red meat will vary depending on the proportions of lean meat and fat present. Lean meat is relatively higher in PUFA and lower in SFA compared with untrimmed meat. Trimming the fat off meat will affect the proportions of fatty acids, as visible fat is relatively higher in SFA, containing about 37 g SFA per 100 g meat( Reference Li, Siriamornpun and Wahlqvist 7 ).
Overall, lean red meat contains similar proportions of MUFA and SFA, although the exact proportions vary depending on the type of meat. Beef and lamb (and other meats from ruminants) generally contain more SFA than pork (or meat from non-ruminants) as the majority (>90 %) of the dietary unsaturated fatty acids are hydrogenated to SFA in the rumen during digestion( Reference Lunn and Theobald 8 ). The main SFA present in red meat are palmitic acid (C16 : 0) (approximately half) and stearic acid (C18 : 0) (approximately one-third). While palmitic acid appears to increase blood cholesterol levels, stearic acid has a neutral effect on total and LDL-cholesterol( Reference Daley, Abbott and Doyle 9 ). Red meat also contains minor amounts of myristic acid (C14 : 0) and lauric acid (C12 : 0) that are thought to increase blood cholesterol more potently than palmitic acid. Both of these fatty acids are present in relatively low amounts, for example, 0·1/100 g myristic acid in lean beef( 10 ).
Red meat also contains relatively low levels of PUFA; however, it can contribute substantially to intakes, providing 18 % of n-6 PUFA and 17 % of n-3 PUFA, while contributing to 23 % of overall fat intake( Reference Henderson, Gregory and Irving 11 ). The main PUFA in red meat are the essential fatty acids, linoleic (n-6) and α-linoleic acid (n-3). When consumed, the body can convert α-linoleic acid into the long-chain beneficial n-3 fatty acids EPA and DHA. The rate of synthesis however is small, with studies generally suggesting conversion rates of α-linoleic acid to DHA of below 5 % in man( Reference Thomas 12 ). Recent work has focused on improving the fatty acid profile of red meat( Reference Scollan 13 ).
Data from the most recent National Diet and Nutrition Survey show trans-fatty acid intakes represented 0·7 % of food energy( 14 ). Industrially produced trans-fatty acids, such as those found in fat spreads, biscuits, cakes, pastries, crisps and confectionery have been the focus for reduction. Meat and dairy products from ruminant animals contain low levels of natural trans-fatty acids. The predominant trans-fatty acid in red meat is vaccenic acid( Reference Turpeinen, Mutanen and Aro 15 ), which has not been associated with CHD( Reference Willett, Stampfer and Manson 16 ). Conjugated linoleic acid is a collective term for a mixture of positional and geometric isomers of linoleic acid. These isomers are intermediates in the biohydrogenation of linoleic acid, with the majority of conjugated linoleic acid produced from vaccenic acid. Evidence from in vitro and animal studies indicates that conjugated linoleic acid has many potential health benefits for cancer, CHD and diabetes( Reference Daley, Abbott and Doyle 9 ) and enhancing immune function and affecting body composition change (reducing fat gain and enhancing lean body mass gain)( Reference Pariza, Park and Cook 17 ). However, the few studies conducted in man are inconsistent and conflicting( Reference Ploudre, Jew and Cunnane 18 , Reference Nakamura, Flintoff-Dye and Omaye 19 ) as very high doses (of 3 g/d) may be required for a beneficial effect( Reference Ploudre, Jew and Cunnane 18 ).
Micronutrients
Red meat provides a wide range of bioavailable micronutrients which are required for general health and wellbeing. For example, most of the iron in meat is in the haem iron form. Single meal studies have shown that haem iron is more efficiently absorbed from the diet (20–30 %) than non-haem iron (5–15 %)( Reference Martinez-Torres and Layrisse 20 , 21 ). The haem iron in red meat also enhances non-haem iron absorption from foods such as cereals, vegetables and pulses consumed at the same time( 22 ) Meat and meat products contribute to 21 % of iron intake in adults (aged 19–64 years)( 14 , Reference Bates, Lennox and Prentice 23 ).
Although there is currently no reference nutrient intake for the UK population aged 4–64 years( 24 , Reference Cashman, Seamans and Lucey 25 ), the UK Scientific Committee for Nutrition has recently prepared a draft review on vitamin D( 14 , Reference Dunnigan and Henderson 26 ). This review recommends a reference nutrient intake for vitamin D of 10 µg/d for the UK population aged 4 years and over( 14 , Reference Dunnigan and Henderson 26 ). Meat and meat products contribute to 35 % of vitamin D intake in teenagers (11–18 years) and 30 % in adults (19–64 years)( 14 , Reference Bates, Lennox and Prentice 23 ). It is thought that the vitamin D in meat is derived from the action of sunlight on the skin of animals or from the animals’ feed( 27 , 28 ). The vitamin D3 metabolite 25-hydroxycholecalciferol (25(OH)D3) is found in significant quantities in meat and liver and is considered to have a high biological activity, resulting in better and faster absorption from the diet compared with its parent compound, cholecalciferol, vitamin D3 ( Reference Groff, Gropper and Junt 29 , 30 ). Data suggest that per microgram vitamin D compound consumed, 25(OH)D3 is approximately five times as effective as vitamin D3 in elevating serum 25(OH)D3 concentration( Reference Cashman, Seamans and Lucey 25 , 31 ). It has also been suggested that components of meat protein may enhance the utilisation of vitamin D in man, particularly where exposure to sunshine is limited( Reference Dunnigan and Henderson 26 , Reference Absoud, Cummins and Lim 32 ).
Many of the micronutrients found in red meat are currently found in low levels in various population groups. According to EU health claims regulation (Regulation (EC) No 1924/2006)( Reference Bates, Lennox and Prentice 23 , 28 ), red meats can be described as a ‘source’ or a ‘rich source’ of several nutrients. In order to make a nutrition claim, the claim in question has to be included in the annex to the Regulation on nutrition and health claims (Regulation (EC) No 1924/2006). A selection of these micronutrients and where they meet criteria for a nutrition claim, for a variety of meats, is presented in Table 1.
Table 1. Selected micronutrients in meats related to nutrition claims classification
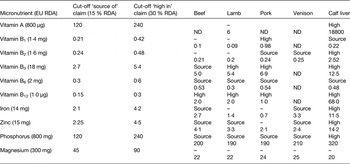
Red meat can make an important contribution to intakes of micronutrients that are sometimes found to be lacking in the diets of some population groups. Table 2 highlights micronutrient intakes that are below the lower reference nutrient intake in different population groups (age and gender) in the UK. Intakes of some micronutrients are found to be below the lower reference nutrient intake across the population and in particular low intakes of the minerals magnesium, iron, potassium and zinc are apparent in adolescent girls. Red meat can make an important contribution to these nutrients. Data from the latest UK National Diet and Nutrition Survey indicate that the contribution of meat and meat products to the average daily intake of magnesium is 15 %, 21 % for iron, 18 % for potassium and 36 % for zinc( Reference Bates, Lennox and Prentice 23 ).
Table 2. Selected micronutrient intakes: percentage with intakes below the lower reference nutrient intake

Source: National Diet and Nutrition Survey (2014)( 14 , Reference Bates, Lennox and Prentice 23 ).
Role of red meat in different population groups
Red meat can play a useful role in providing nutrients to the general population. The present paper briefly discusses the role of red meat in providing some key nutrients in the diet of young infants, adolescents, women of child-bearing age and older adults.
Red meat can provide an important contribution to micronutrient intakes among young infants, particularly around the time of weaning. At around 6 months, stores of some nutrients, such as iron, start to be depleted and therefore additional dietary sources are required. In practice, infants are often weaned initially onto foods such as baby rice, fruits and vegetables, with the introduction of meat often being delayed until 8 or 9 months, which can impact on iron intakes. This is particularly true where mothers continue to breastfeed rather than give formula or follow-on milk, which is fortified with iron. Although iron can be obtained from other sources, red meat provides an important source of highly bioavailable iron. Young children should receive a variety of foods including meat, as soon as possible( 27 , 31 ).
Gender differences can be seen among young children (aged 4–10 years) and adolescents (aged 11–18 years) with low micronutrient intakes more common among girls (Table 2). One reason for this may be that boys tend to consume more breakfast cereal, full-fat milk, meat and meat products than girls. Vitamin D insufficiency is widespread amongst many population groups in the UK, including children( Reference Groff, Gropper and Junt 29 , Reference Absoud, Cummins and Lim 32 ). The average daily intake of vitamin D is 2·1 µg among 11–18 year olds( 14 , Reference Bates, Lennox and Prentice 23 ).
The diet and nutrient intake of women of childbearing age and around the time of pregnancy is important for the women's health as well as for the health off her offspring and future generations. Vitamin D and iron are of particular importance around pregnancy. Maternal vitamin D status may be critical to bone development in the offspring( Reference Quincey, Dennison, Cooper, Wyness, Stanner and Buttriss 33 ). Many women in the UK have low vitamin D blood levels, especially in winter and early spring. A serum/plasma 25-hydroxyvitamin D (25-(OH)D) concentration of below 25 nm/l is currently used to indicate low vitamin D concentrations( 24 , Reference Cashman, Seamans and Lucey 25 ). A cohort study of pregnant women in Northwest London (n 346)( Reference McAree, Jacobs and Manickavasagar 34 ) reported that the proportion with a plasma 25(OH)D concentration below 25 nm/l was 49 % in winter and 29 % in summer. Iron intakes among non-pregnant women of childbearing age are frequently below recommended levels (Table 2) and although there is no national data on iron intakes or prevalence of iron deficiency in pregnant women in the UK, several small British studies suggest that intakes below reference levels are evident( Reference Buttriss, Stanner, Sanders, Wyness, Stanner and Buttriss 35 ).
Ageing causes gradual reductions in skeletal muscle mass, known as sarcopenia or sarcopenic obesity (replacement of lost skeletal muscle with fat)( Reference Frontera, Zayas and Rodriguez 36 ). It is well established that sarcopenia is a geriatric syndrome which is common within the UK( Reference Patel, Syddall and Jameson 37 ) and can be prevented or progression delayed through targeted nutrition and lifestyle advice. Increased consumption of high-quality protein during middle age and beyond is necessary to maintain the quality of life associated with adequate muscle mass and strength. It has been suggested that current protein recommendations may not be sufficient to prevent sarcopenia in elderly people( Reference Phillips 38 ). The branch-chain amino acids (leucine, isoleucine and valine) are the essential amino acids needed for protein synthesis and these are generally higher in animal proteins than plant proteins, with the highest levels found in red meat( Reference McNeill 39 ). Meat proteins may be more effective in preventing sarcopenia than soya( Reference Phillips 38 ). Thus, including red meat in the diet of older adults could help delay sarcopenia, which is a common cause of physical disability( Reference Janssen, Shepard and Katzmarzyk 40 ).
Satiety and weight control
There has been a great interest on the impact of various aspects of the diet on satiety and body weight and the role of protein in modulating appetite and energy intake has been the subject of a number of studies. Protein tends to be more satiating than other macronutrients, both at the level of a single eating occasion and over days and weeks( Reference Paddon-Jones, Westman and Mattes 41 ). There is clear evidence from short to medium term studies that ad libitum high-protein diets increase satiety and increase weight loss compared with high-carbohydrate diets( Reference Skov, Toubro and Rønn 42 , Reference Weigle, Breen and Matthys 43 ). This effect seems to persist to a small degree in the longer term (over 12 months)( Reference Clifton, Condo and Keogh 44 ). When comparing two types of protein sources (meat and soya) in a high-protein meal and diet, the effect on appetite was found to be similar( Reference Douglas, Lasley and Leidy 45 , Reference Neacsu, Fyfe and Horgan 46 ). When different types of meat were considered, evidence also suggests similar effects on appetite control. No differences were found in the release of intestinal hormones associated with appetite and hunger signalling, nor in subjective measures of appetite and hunger over a 3-h period following consumption of beef, pork or chicken( Reference Charlton, Tapsell and Batterham 47 ). It is important to note that in a study where macronutrient content of test meals was varied while energy density remained constant, no differences in satiety or subsequent energy intake were observed( Reference Raben, Agerholm-Larsen and Flint 48 ). It may be that energy density of a food or diet may be more important in determining its satiating effect than its protein content( Reference Wyness, Weichselbaum and O'Connor 49 ).
How much red meat should we eat?
In response to the findings of the Scientific Advisory Committee on Nutrition report on Iron and Health( 22 ) the UK Department of Health issued new guidance in 2011 on eating red and processed meat. The Scientific Advisory Committee on Nutrition found evidence that there is a probable link between eating a lot of red and processed meat and increased risk of colorectal cancer incidence. Hence, the Department of Health's advice is that adults who eat more than 90 g red and processed meat daily should reduce their intake to an average of 70 g/d (cooked weight)( 50 ). The current average UK consumption of red and processed meat is 86 g/d among men and 56 g/d among women( Reference Bates, Lennox and Prentice 23 ).
Processed red meat
The composition of processed meat can vary widely. The salt content of processed meats is likely to be higher than lean red meat. Salt is often added to processed meats to reduce the amount of available water present in the food, and as a result, micro-organisms grow more slowly or not at all. Over the past several years, there has been considerable work done within the UK to reduce the amount of salt in processed meat products, along with consideration of the potential effects on microbial safety( Reference Wyness, Stanner and Buttriss 51 ). Meat and meat products contribute to 27 % of sodium intake in adults (aged 19–64 years) which is just below the contribution of 31 % that cereals and cereal products make to sodium intakes in adults( Reference Bates, Lennox and Prentice 23 ).
Conclusions
Red meat provides a rich source of high-quality protein and a variety of important nutrients that are vital for optimal health. The nutrients in red meat can provide a useful contribution to the intakes of key nutrients commonly found to be in short supply in the diets of some population groups. In particular, the nutrient intakes of young infants, adolescents, women of child-bearing age and older adults may benefit from including lean red meat in their diets. Lean red meat may be a useful component of weight loss diets because of the satiating effect of its high protein content. Whether the protein is from meat or non-meat sources does not appear to have a significant effect on satiety in the context of a normal diet. Currently, the average UK consumption of red and processed meat for women falls below, and for men, falls slightly above the figure advised by the Department of Health. Some men, in particular those who are high consumers of red and processed meat, should reduce their intake to meet 70 g/d on average advised by the UK Government.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The author was solely responsible for all aspects of preparation of this paper.





