From the early use of saccharin over 100 years ago to the present day, low-calorie sweeteners (LCS) have been subject to much critical comment and disagreement. In large part, this has to do with consumers’ concerns about their safety, which persist despite LCS being among the most thoroughly tested and evaluated food additives(Reference Roberts1). Those concerns have led to a potential market for LCS of ‘natural’ origin, such as steviol glycosides, though presently, acesulfame-K, aspartame and sucralose, along with saccharin, are the most widely used LCS. The potential benefits for public health of LCS are a reduction in sugar intake and consequent reduction in the prevalence of obesity and dental caries. These are the primary reasons for the use and further development of LCS in foods, beverages and products such as chewing gum, toothpaste and medicines. Nonetheless, the role of LCS in weight management is controversial, with claims that LCS consumption may increase rather than decrease the risk of overweight and obesity. The purpose of the present review is to examine the evidence for and against these claims, starting with a brief account of our attraction to sweetness.
(The author uses the term sugar to refer to sugars in general. In the research that is cited, the sugars are mostly sucrose, fructose, glucose and high-fructose maize syrup.)
Sweetness
Human beings have an inborn and universal liking for and acceptance of sweetness. This is evident from, for example, the positive affective reactions elicited in human newborns by placing a small amount of sucrose solution into their mouths, which is in stark contrast to the distress and rejection caused by bitter-tasting substances(Reference Steiner, Glaser and Hawilo2). The dislike and rejection of bitterness are thought to provide protection against ingestion of plant toxins, especially alkaloid compounds most of which are bitter tasting. The function of our liking for sweetness is perhaps somewhat less clear, however. The usual argument is that sweetness signals energy in the form of sugar, but, as described later, the most readily available sources of sugar pre-industrially (i.e. fruit and berries) are less energy dense than non-sweet carbohydrate sources (e.g. roots and tubers; Table 1). Nonetheless, fruit and berries are significant sources of energy (and micronutrients), and can be consumed without cooking. Furthermore, this energy source detection hypothesis is supported by the intriguing finding that, over their evolutionary history, cats have lost the ability to detect sweet taste, presumably because as they became obligate carnivores they no longer had a need to detect sugar(Reference Beauchamp3). Indeed, the loss of sweet taste function may have facilitated their path to full carnivory. The example of cats also provides evidence against the primary function of sweetness being to motivate consumption of our and other mammals’ first food, namely lactose-containing milk(Reference Beauchamp3, Reference Booth, Higgs and Schneider4). For plants, the function of sweetness appears to be seed dispersal(Reference Jordano, Forget and Lambert5). Plants ‘want’ their fruit or berries to be eaten, and manipulate frugivorous behaviour through changes in sourness, sweetness, colour and other cues timed to coincide with their seeds’ ripeness. Some plant species have even developed highly sweet-tasting proteins such as brazzein to entice seed dispersers(Reference Guevara, Veilleux and Saltonstall6), presumably with the advantage to the plant that the metabolic cost per unit of sweetness is lower for brazzein than for sugar. A function of bitterness for plants is a defence against the predation of their leaves and stems.
Table 1. Energy, sugar and total carbohydrate content per 100 g of some ‘natural’ (i.e. minimally processed) carbohydrate-rich foods
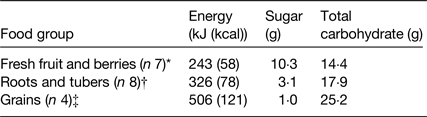
* Apple, banana, blueberries, grapes, pear, orange, strawberries.
† Carrot (raw), carrot, cassava, parsnip, potato, sweet potato, turnip, yam. Boiled in water except for raw carrot.
‡ Couscous, maize, pasta, rice. Boiled in water.
The example of brazzein, consumption of saccharin solutions by rats(Reference Smith and Sclafani7) and sales of LCS beverages and chewing gums, all show that sweetness without energy is sufficient to motivate consumption. In other words, sweetness alone is rewarding. In the context of higher than recommended levels of sugar consumption and high prevalence of overweight and obesity, this is encouraging for the use of LCS. The question is then, to what extent does replacing some of the added sugar in the diet with LCS reduce overall energy intake and body weight? In the following sections, the author considers this question by examining: (1) short- and longer-term influences on appetite; (2) the evidence concerning specifically the effects of LCS on energy intake and body weight; (3) arguments as to why the use of LCS might be counterproductive to healthy weight management.
Energy balancing and the potential usefulness of consuming sweetness without energy
Energy intake meal to meal is influenced primarily by the opportunity to eat, including habit (e.g. it is lunchtime or mid-afternoon snack time), and the acute satiating effect of food sensed in the gut during consumption and soon afterwards(Reference Rogers and Brunstrom8). By contrast it is only weakly influenced by longer-term energy balance(Reference Rogers and Brunstrom8). These dynamics of human appetite and weight control are illustrated in Fig. 1.
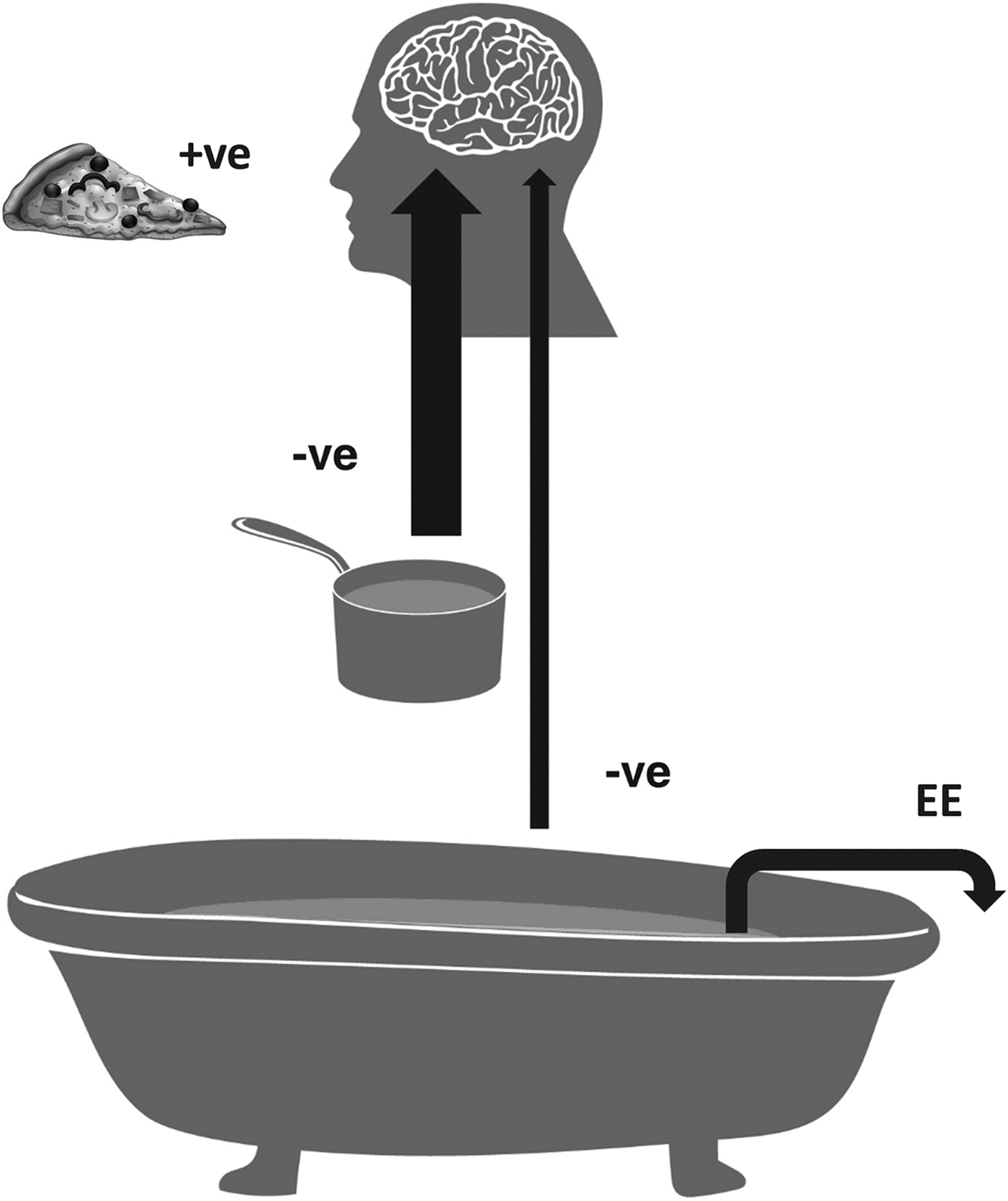
Fig. 1. The saucepan and bathtub analogy for human appetite and weight control, adapted from Rogers and Brunstrom(Reference Rogers and Brunstrom8). This is an incentive model of appetite in which, for the well-nourished individual, eating, resulting in energy intake, is motivated by the anticipation of food reward (loosely the pleasure of eating). By default, eating is rewarding, and is inhibited by fullness caused by food intake and by increased body energy stores. More specifically, the slice of pizza represents the stimulatory effect of liked food on eating, the water in the saucepan represents food in the gut and the water in the bathtub represents body energy stores. The bathtub is filled via the saucepan. It takes several hours for the energy content of the saucepan to move to the bathtub. Both the saucepan and bathtub resist filling, representing negative feedbacks on eating (i.e. respectively, strong acute and weak chronic inhibitory signals). The arrow labelled EE represents energy expenditure. The model recognises that the change in energy balance from one meal to the next is trivial compared with the amount of energy stored in the body and readily available to maintain energy supply to the body's organs and tissues if a meal or even several meals are missed. However, food intake needs to be controlled because the limited capacity of the gut means that processing a meal poses a significant physiological challenge(Reference Woods9). The model is consistent with the observations that appetite is reduced acutely by energy intake (a meal added to the limited capacity of the saucepan/gut), but largely unaffected by an acute increase in energy expenditure (energy removed from the large store of energy in the bathtub/body)(Reference Schubert, Desbrow and Sabapathy10). The existence of a relatively weak but chronic negative feedback effect on appetite proportional to body fatness is supported by observations on the dynamics of changes in energy intake and body weight in rat dietary obesity(Reference Mela and Rogers11–Reference Rogers13) and in human participants challenged with the covert imposition of negative energy balance(Reference Polidori, Sanghvi and Seeley14).
The energy content of a meal is a major, although by no means the sole determinant of its satiating effect(Reference Rogers and Brunstrom8). Furthermore, the inhibitory effects of food intake on appetite decline rapidly during the inter-meal interval, so that even after a large meal we are ready to eat again within a few hours (and often before our energy expenditure during that period exceeds the energy consumed in the meal). For example, our appetite for lunch and capacity for eating lunch is very similar whether or not we have eaten breakfast(Reference Levitsky and Pacanowski15, Reference Rogers, Ferriday and Jebb16). It follows, therefore, that if a meal is missed or significantly postponed, overall daily energy intake is likely to be reduced(Reference Levitsky and Pacanowski15). More subtly, consuming a smaller meal or a reduced-energy meal also ought to contribute to reduced overall energy intake.
One way of reducing the energy content of a meal, or in fact the whole diet, is to (partially) replace sugar with LCS. Compared with merely consuming less, a potential advantage of using LCS is that they preserve the sweetness of the meal or diet and thereby maintain the pleasure of consumption(Reference Rogers and Brunstrom8). There are, nevertheless, obvious (and perhaps less obvious) limits to the reduction in energy intake and body weight that can in practice and in theory be achieved with LCS. First, of course, there is the amount of sugar consumed in the diet. If this is a fairly small quantity there is clearly less scope for reduction than if sugar intake is high, and especially if a large proportion of that sugar intake is from beverages, as generally more sugar can be replaced with LCS in beverages than in foods(Reference Gibson, Ashwell and Arthurs17). Second, there is distrust of LCS among some consumers (and health professionals), which leads to avoidance of LCS-containing foods and beverages. Paradoxically, distrust of LCS appears to be partly founded on concerns that consumption of LCS might increase energy intake and body weight. Third, there are the dynamics of appetite and weight control. Although, as described earlier, dilution of energy density with LCS can be expected to reduce energy intake and therefore over time reduce body weight, the reduction in body weight will be constrained. This is because as weight loss ensues the inhibitory effect of body fat stores on appetite diminishes (Fig. 1), causing energy intake to begin to increase a little (i.e. the deficit in energy intake reduces). At the same time, there will be a small decrease in energy expenditure associated with the loss of body weight. Together, all else being equal, these effects will cause weight settle (plateau) at a new lower level(Reference Mela and Rogers11–Reference Polidori, Sanghvi and Seeley14). How much lower will depend on the extent of the initial reduction in energy intake achieved by the use of LCS. These dynamics apply to any intervention that successfully affects energy intake or energy expenditure. They also help explain why weight is so often regained after weight loss. When the intervention is removed, weight moves towards and eventually settles at the equilibrium point characteristic of the pre-intervention eating and physical activity environment.
In summary, use of LCS can be expected to reduce sugar and energy intakes and thereby contribute to healthy weight management. What then is the evidence, if any, in support of this?
Evidence from human and animal studies
Recent meta-analyses of acute and longer-term randomised controlled studies in human participants found clear evidence that consumption of LCS compared with sugar does indeed reduce energy intake and body weight(Reference Miller and Perez18, Reference Rogers, Hogenkamp and de Graaf19). For example, many so-called preload test-meal studies measuring the effects of LCS v. sugar in foods and beverages have been performed. This method tests the effect of consuming a fixed portion of food or beverage (the preload) on energy intake in an ad libitum test-meal served at a fixed interval after the preload. The meta-analysis results showed that for adults and children (n 1319) test-meal energy intake was higher after the LCS v. the sugar preload(Reference Rogers, Hogenkamp and de Graaf19). However, the higher intake compensated for only 50% of the energy difference between the preloads. That is, cumulative energy intake (preload + test-meal) was lower, at an average of 393 kJ (94 kcal), after the LCS preload(Reference Rogers, Hogenkamp and de Graaf19). It is also noteworthy that this demonstrates that sugar in liquids is not somehow ‘missed by the body’. Indeed, in a direct comparison, partial compensation for sugar (v. LCS) was found not to differ between a beverage and semi-solid and solid foods(Reference Gadah, Kyle and Simth20).
While these results favour the consumption of LCS in place of sugar (when reduced sugar and reduced overall energy intake are desirable), there is some uncertainty about how they translate into real life outside the laboratory. For example, perhaps energy intake compensation increases with repeated exposure to LCS. Also there is evidence that carry-over effects inherent in the cross-over designs used in these studies cause compensation to be underestimated(Reference Gadah, Brunstrom and Rogers21). Another possibility is that there might be a further adjustment (compensation) in energy intake after the test-meal. However, there is evidence against this. For example, Levitsky and Pacanowski(Reference Levitsky and Pacanowski15) found that participants ate 565 kJ (135 kcal) more at lunch when they missed breakfast compared with when they ate a 2615 kJ (625 kcal) breakfast (i.e. 22% compensation), but there were no further differences in energy intakes in subsequent snacks and meals during the rest of the day. As indicated in the previous section, this also supports a declining influence of an energy deficit (no breakfast v. breakfast, or LCS v. sugar) across the inter-meal interval. Indeed, for that reason it could be argued that because a majority of the preload studies used a preload to test-meal interval of an hour or less, 50% compensation overestimates the compensation that occurs at longer inter-meal intervals in real life.
Given these difficulties in estimating real-life effects of LCS consumption from short-term preload test-meal studies, it is also valuable to have evidence on the effects of LCS v. sugar from longer-term (randomised-controlled) intervention studies. Systematic reviews, including meta-analyses, of these studies show that LCS compared with sugar reliably reduces energy intake and body weight(Reference Miller and Perez18, Reference Rogers, Hogenkamp and de Graaf19). For the most recent meta-analysis of effects on body weight participants were adults and children (n 1332) and the duration of the intervention and any follow-up varied from 4 weeks to 40 months(Reference Rogers, Hogenkamp and de Graaf19). Outcomes were similar for studies in which the test products, mostly beverages, were added to the diet and those in which the participants were already consuming sugar-sweetened products and the intervention was (partial) replacement of sugar with LCS(Reference Rogers, Hogenkamp and de Graaf19). The effect sizes of LCS v. sugar were −1·41 (95% CI −2·62, −0·20) kg for adults and −1·02 (95% CI −1·52, −0·52) kg for children. The studies included in this review(Reference Rogers, Hogenkamp and de Graaf19) are the same as some of the studies that have been cited as demonstrating that consumption of free sugars increases body weight(Reference Te Morenga, Mallard and Mann22). Additionally, the finding that iso-energetic exchange of sugars with other carbohydrates does not change body weight indicates that this effect is not specific to sugar(Reference Te Morenga, Mallard and Mann22). In other words, it is the difference in energy density of the diet that affects body weight (fatness).
We also reviewed evidence from prospective cohort studies and animal studies(Reference Rogers, Hogenkamp and de Graaf19). There was no overall association between LCS consumption and body weight in the prospective cohort studies, although the largest such study (125 000 adult participants from three cohorts) found a small significant association in the direction of reduced weight with LCS consumption(Reference Pan, Malik and Hao23). One smaller, often cited, study also in adults (n 3371) found weight to be significantly positively associated with LCS consumption(Reference Fowler, Williams and Resendez24). Given the results of the intervention studies, we concluded that this evidence from cohort studies is likely explained by the presence of reverse causation and confounding(Reference Rogers, Hogenkamp and de Graaf19).
In a large majority of studies in which animals (mice and rats) have been exposed to LCS and data on body weight were collected, LCS were found to reduce weight or have no effect(Reference Rogers, Hogenkamp and de Graaf19). The main purpose of many of these studies was to test the safety of LCS, although this group of studies also included some in which LCS were used as a control in tests of the effects of sugar on energy intake and body weight. By contrast, a large majority (nineteen out of twenty-two) of the studies, mainly from the same research group, which used a specific procedure of intermittent exposure to food supplemented with glucose or LCS(Reference Swithers, Martin and Davidson25), found that weight increased more in the rats receiving LCS(Reference Rogers, Hogenkamp and de Graaf19). This research on intermittent exposure to LCS in rats has been widely cited by critics questioning the usefulness of LCS for weight management(Reference Hampton26–Reference Yang28). In the next section, the author examines the rationale for these studies and summarises very recent work that contradicts the original authors’ conclusions. In the subsequent two sections, the author examines two further arguments critical of LCS.
Conjecture: low-calorie sweeteners disrupt the learned control of energy intake (sweet taste confusion hypothesis)
Swithers et al. (Reference Swithers, Martin and Davidson25) set out the premises for their studies investigating the effects of intermittent exposure to LCS as follows (numbering added) (1) ‘We reasoned that if sweet tastes are normally valid predictors of increased caloric outcomes, (2) then exposing rats to sweet taste that is not associated with these outcomes should degrade this predictive relationship, (3) impair energy intake and body weight regulation’. They also stated that (4) ‘In nature, and throughout most of our evolutionary history, sweetness has been a reliable predictor of the energy content of food’. In the studies, rats were given continuous ad libitum access to (slightly sweet) laboratory chow, and additionally they were fed a fixed portion of sweetened yoghurt on 3 d/week and unsweetened yoghurt on another 3 d/week. For one group of rats the sweetener was glucose and for another group of rats it was saccharin, making sweetness respectively predictive and not predictive of an increase in yoghurt energy density. The rats consumed all or nearly all the yoghurt offered. Over a period of several weeks the saccharin-fed rats were found to have higher overall energy intake and gain more weight than the glucose-fed rats(Reference Swithers, Martin and Davidson25).
Recent research, however, has failed to replicate these results of intermittent exposure to saccharin v. glucose. Using the same paradigm as Swithers et al. (Reference Swithers, Martin and Davidson25), Boakes et al. (Reference Boakes, Kendig and Martire29) report the opposite result, namely that the glucose-fed rats gain the most weight (body fat). The discrepancy in the results appears to be explained by a crucial difference in procedure; Swithers et al. (Reference Swithers, Martin and Davidson25) having excluded individual rats that showed low acceptance of the saccharin-sweetened yoghurt(Reference Boakes, Kendig and Martire29, Reference Swithers30). Boakes et al. (Reference Boakes, Martire and Rooney31) demonstrate that this biases the sample towards fast-growing individuals, as saccharin acceptance is positively associated with later weight gain on laboratory chow. It appears therefore that a crucial piece of evidence used to support the claim that LCS disrupt the learned control of energy intake is a procedural artefact. The finding that rats fed glucose-sweetened yoghurt gained more weight is plausibly explained by a lack of full compensation for the higher energy content of the glucose-sweetened yoghurt(Reference Boakes, Kendig and Martire29). Relatedly, another recent study found that exposing rats to a large variety of highly processed foods did not impair flavour-nutrient learning, compared with exposure to either a variety of minimally processed foods or a standard chow diet(Reference Palframan and Myers32). The authors conclude that their results contradict the flavour-confusion hypothesis.
In any case, notwithstanding these differing conclusions, there is a problem with the reasoning that sweetness could be a useful guide for the control of energy intake, if it were not for the disruptive effect of LCS. This is simply because, even when foods and beverages containing LCS are excluded, the level of sweetness does not reliably predict the energy content of different foods and beverages in the diet. The results summarised in Table 2 show that, not surprisingly, sweetness predicts the sugar content of products whereas, crucially at the same time, sweetness and energy content are essentially unrelated. So, it is incorrect to assume (reason) that, at least for modern human beings, sweet tastes are normally valid predictors of increased caloric outcomes(Reference Swithers, Martin and Davidson25). Furthermore, the statement that ‘in nature, and throughout most of our evolutionary history, sweetness has been a reliable predictor of the energy content of food’ is also invalid. Comparing natural foods, that is, foods that require at most only basic processing before consumption, it is again clear that sweetness cannot be relied upon as a guide to energy content. Among the categories of carbohydrate-rich foods shown in Table 1, sugar content (a proxy for their sweetness) is, if anything, inversely related to energy density.
Table 2. Correlations (Pearson's r) between sweetness and sugar and energy content of foods and beverages in three studies

Actually, for oro-sensory control of energy intake to be effective in the modern world, it would seem that what needs to be learned is the relationship between a configuration of oro-sensory cues and post-ingestive consequences. For example, take two desserts, orange sorbet and chocolate ice cream. The sorbet contains more sugar and is highly sweet, whereas the ice cream is moderately sweet (and is creamy and thicker), but owing to its substantial fat content it is the more energy dense dessert. So among desserts, levels of sweetness, creaminess and/or viscosity together help predict energy density, whereas sweetness alone is a poor predictor of energy density, and probably poorer than, for example, creaminess or viscosity alone.
In summary, it appears that LCS can be absolved from the charge that they disrupt the learned control of appetite. The case for this effect falls at the first hurdle because sweetness is not a reliable predictor of energy density, including among minimally processed foods and when LCS foods and beverages are disregarded. Furthermore, even when sweetness is set up to be a reliable cue for increased energy density, the evidence indicates that this does not protect against weight gain(Reference Boakes, Kendig and Martire29). Instead, energy density itself (rather than any learning about energy density) dominates as the influence on body weight, so that weight is lower when the food is sweetened with LCS than when it is sweetened with sugar(Reference Boakes, Kendig and Martire29). Of the premises quoted earlier(Reference Swithers, Martin and Davidson25), 2 is logically correct, but 1, 3 and 4 are incorrect.
Conjecture: exposure to sweetness increases desire for sweetness (sweet tooth hypothesis)
Another argument against the consumption of LCS is that exposure to sweetness encourages a sweet tooth and therefore increased intake of sweet, energy-containing foods and beverages. Examples of this argument are: ‘over-stimulation of sugar receptors by frequent consumption of hyper-intense sweeteners may cause taste preferences to remain in, or revert to, an infantile state’(Reference Ludwig27), and ‘artificial sweeteners, precisely because they are sweet, encourage sugar craving and sugar dependence’(Reference Yang28). A more measured statement is that ‘repeated exposure to non-nutritive sweeteners, i.e. LCS, would be expected to establish and maintain a preference for sweet items in the diet’(Reference Mattes and Popkin36). There is, though, little direct evidence to support these statements. Indirectly, there is substantial evidence that taste and flavour preferences can be increased through repeated exposure. Preference for salt is a good example, with studies demonstrating that increased oro-sensory exposure to salt in food increases preference for higher concentrations of salt, with decreased exposure having the opposite effect(Reference Mattes and Popkin36). Moreover, after 2001 there was a step-wise reduction in the salt content of foods in the UK and a concomitant decline in salt intake(37).
But is salt reduction a good analogy for sugar reduction? If it were, consumption of water (non-sweet) in place of sugar-containing beverages ought to decrease energy intake and body weight more than replacing the sugar with LCS, because the preference for sugar, in general, would decline. In preload test-meal studies, water and LCS beverages have been found not to differ in their effects on test-meal energy intake(Reference Rogers, Hogenkamp and de Graaf19). The same has been found for equi-energetic LCS-sweetened v. non-sweet food preloads(Reference Rogers, Hogenkamp and de Graaf19). This, of course, could reflect the short-term nature of exposure to sweetness in these studies. More tellingly, though, the evidence from longer-term intervention studies on body weight, if anything, favours LCS over water(Reference Rogers, Hogenkamp and de Graaf19). This may be explained, at least in part, by the difficulty of having to give up sweet beverages. In the study showing the largest effect(Reference Peters, Wyatt and Foster38), participants were consumers of LCS beverages enrolled in a behavioural weight loss programme and randomised to continue to consume LCS beverages or water. So perhaps it was easier to comply with the programme if it was not also necessary to stop consuming LCS beverages. The more relevant question is whether there is an advantage in switching from sugar-sweetened beverages to LCS-sweetened beverages v. switching to water. The one study (the CHOICE trial) that has looked at this found slightly, but not statistically significant, greater weight loss in the group switching to LCS(Reference Tate, Turner-McGrievy and Lyons39).
The CHOICE trial also found that consumption of LCS beverages v. water led to a reduction in energy intake from sugars and desserts(Reference Piernas, Tate and Wang40). This contradicts the sweet tooth hypothesis, instead indicating that exposure to LCS may satisfy rather than increase desire for sweetness. Such an effect is consistent with the phenomenon of sensory-specific satiety, which describes the short-term reduction in liking or reward value of a recently consumed food or taste(Reference Rolls, Hetheringtom and Burley41, Reference Rogers and Hardman42). We tested this directly for LCS. We found that within a meal, consumption of a LCS beverage v. water reduced rather than increased desire for, and intake of, sweet food relative to non-sweet food (P. J. Rogers et al., unpublished results). In another study, participants who reduced their intake of sweet foods and beverages for 3 months showed an increase in perceived sweet-taste intensity (at low concentrations of sucrose) but no change in perceived pleasantness of sweet test products(Reference Wise, Nattress and Flammer43). Similarly, adults’ preference for sweet orangeade and sweet yoghurt were not affected by 8 d exposure to the sweet orangeade, although there was some evidence that exposure increased sweetness preference in children(Reference Liem and de Graaf44). (A systematic review of the effect of sweet taste exposure on acceptance and preference for dietary sweetness is presently being undertaken(Reference Appleton, Mela and Bertenshaw45)).
Taken, together, these quite different studies indicate that consumption of LCS beverages does not increase energy intake compared with water, and may have the advantage of to some extent satisfying desire for sweetness when consumed shortly before or with a meal. A caveat to this conclusion is that another study comparing replacement of LCS beverages with water v. continuing to consume LCS beverages found greater weight loss in the water group(Reference Madjd, Taylor and Delavari46). An explanation as to why these results differ from the study by Peters et al. (Reference Peters, Wyatt and Foster38) may lie in the procedure of permitting only one LCS beverage to be consumed daily, after lunch(Reference Madjd, Taylor and Delavari46). Possibly, this prevented consumption of the LCS beverage displacing the consumption of sweet food, as it may have done if consumed shortly before or with the meal. It is unclear, however, how consuming just one LCS beverage daily for 5 d weekly could interfere with weight loss. If it does, this unusual pattern of LCS consumption could be avoided. Finally, by contrast, a follow-up to the Peters et al. (Reference Peters, Wyatt and Foster38) cohort showed that the effect on weight favouring LCS v. water was increased after 1 year(Reference Peters, Beck and Cardel47).
Conjecture: consumers consciously overcompensate for ‘calories saved’ by using low-calorie sweeteners (conscious overcompensation hypothesis)
As described earlier, in preload test-meal studies there is partial but not full compensation in subsequent energy intake for the difference in energy content of LCS- v. sugar-sweetened foods and beverages(Reference Rogers, Hogenkamp and de Graaf19). This demonstrates satiety generated by the post-ingestive detection of sugar, as participants were blinded to the nutrient difference between the preloads. That is, the LCS and sugar preloads were matched for appearance, flavour and taste and, with only a few notable exceptions (described later) participants were not told that the preloads differed. However, in the real world, many food and beverage products sweetened with LCS are identified explicitly as being low energy, diet, zero sugar, slimline, etc., so consumers will likely be aware of consuming a relatively low-energy product. Indeed, very often it will have been their conscious choice to do so. A possible consequence is that this leads the consumer to choose and consume more of the low-energy item, or more of another item, or both, with the result that overall energy intake is unaffected. Or, as suggested by Mattes and Popkin(Reference Mattes and Popkin36), there might be overcompensation, resulting in an overall increase in energy intake.
Perhaps unsurprisingly, information that increases the perceived healthiness of a food has been shown to reduce estimates of its energy content and increase the amount consumed(Reference Provencher, Polivy and Herman48). Conversely, providing information about the energy content of meals on menus together with interpretive or contextual information decreases energy intake(Reference Sinclair, Cooper and Mansfield49). Specifically in relation to LCS, several studies have compared the effect of LCS v. sugar on energy intake in participants informed v. not informed (or correctly v. incorrectly informed) about the sweetener and/or energy content of the manipulated food or beverage. This fairly heterogenous set of studies is summarised in Table 3. None of the studies found that information significantly modified the effect of LCS v. sugar on energy intake. Mattes(Reference Mattes51) concludes from his study that there was a ‘strong tendency for an effect (of information) on intake’. In this cross-over study, participants were fed breakfast cereals which were unsweetened, sweetened with aspartame or sweetened with sucrose. These different versions of corn-flake cereal were eqi-energetic. Half of the participants were informed about the sweetener content and the other half were not informed. Mattes’ conclusion refers to the finding that daily energy intake was 937 kJ (224 kcal) higher in informed participants when they received aspartame- than when they received sucrose-sweetened cereal. This difference was not statistically significant. The difference for uniformed participants was 293 kJ (70 kcal). While this may be a noteworthy(Reference Mattes and Popkin36) finding, this is a small study and no such trend has been observed in other similar studies (Table 3). Conversely, none of the studies summarised in Table 3 confirmed whether the participants attended to the information presented (and mostly it is unclear exactly what participants were told). The null results could therefore be explained by a lack of salience of the sweetener and/or energy content label or other information, rather than by a lack of conscious compensation based on that information.
Table 3. Studies comparing the effect of low-calorie sweeteners (LCS) v. sugar on energy intake in participants informed v. not informed about the sweetener and/or energy content of the manipulated food or beverage
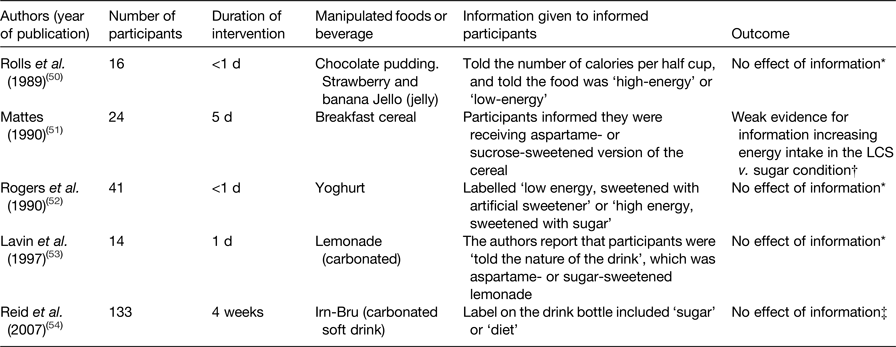
* Effect of information on the difference in energy intake between LCS and sugar conditions.
† The LCS (aspartame) and sugar cereals were equi-energetic.
‡ Half of the participants were correctly and half were incorrectly informed about the sweetener present in their version of the drink.
In a majority of longer-term studies, the LCS v. sugar intervention has been concealed(Reference Rogers, Hogenkamp and de Graaf19). The outcome of those studies, however, does not differ overall from studies in which participants were not blinded to the intervention(Reference Tate, Turner-McGrievy and Lyons39, Reference Blackburn, Kanders and Lavin55), indicating that in the context of attempted weight loss the effect of LCS is not undermined by awareness of LCS consumption. However, further research would be useful. To date, no long-term study has directly compared weight loss in participants (correctly) informed v. not informed about their allocation to consume LCS v. sugar.
In summary, there is little evidence for conscious compensation for LCS consumption. Studies however have not modelled all everyday life uses of LCS. For example, while there might be little or no conscious compensation when LCS are substituted for sugar as part of ‘calorie-counted’ weight loss diet, full or even overcompensation may occur when the choice of LCS is used as an excuse for indulgence. Finally, with certain products or on certain consumption occasions in real life, consumers may be unaware that they are consuming LCS; so under these, perhaps frequent, circumstances conscious compensation can be ruled out as an influence on overall energy intake.
Conclusions
Intervention studies demonstrate that consumption of LCS in place of (some) sugar in the diet reduces energy intake and body weight. Contrary to this evidence are claims that LCS may undermine healthy weight management, and these claims have helped fuel consumer and professional distrust of LCS. Examination of three such claims finds little or no evidence in their support. Most prominent is the claim that LCS consumption undermines the learned control of energy intake; however, this is based on false assumptions and results confounded by a procedural artefact. At the very least, it appears that any counterproductive effects of LCS are outweighed by incomplete compensation for the reduced energy content of LCS foods and beverages.
Acknowledgements
The ideas relating food reward, post-prandial satiety and energy balance were developed in part during the preparation of a grant funded by BBSRC DRINC (BB/L02554X/1). The author thanks Dr David Mela (Unilever, Vlaardingen, The Netherlands, and former colleague of the author at the BBSRC Institute of Food Research, Reading) for proposing that they undertake a systematic review of the evidence on low-calorie sweeteners and weight management, and the author thanks him and his fellow authors for their very substantial contributions to that work.
Financial Support
Part of the author's current research is funded by the European Union Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 607310.
Conflicts of Interest
The author has received funding from Sugar Nutrition UK for research on sugar and satiety, provided consultancy services for Coca-Cola Great Britain and received speaker's fees from the International Sweeteners Association and the Global Stevia Institute. The expert group undertaking the systematic review of low-calorie sweetener consumption and energy intake and body weight(Reference Rogers, Hogenkamp and de Graaf19) received support from ILSI Europe. Preparation of the present review was not supported by any of these organisations.
Authorship
The author had sole responsibility for all aspects of preparation of this paper.






