Iodine (as iodide) is unevenly distributed in the Earth's environment. Leaching from glaciation, flooding and erosion have depleted surface soils of iodide in many regions and most iodide is found in the oceans. In natural cycles, oceanic iodide ions are oxidised to elemental iodine, which volatilises into the atmosphere and is returned to soils by rain(Reference Zimmermann1). However, iodine cycles are often slow and incomplete, leaving soils and drinking-water depleted of iodine. Crops grown in these soils will be low in iodine, and human subjects and animals consuming food grown in these soils become iodine deficient. Iodine-deficient soils are common in mountainous areas and areas of frequent flooding. Many inland areas, including central Asia and Africa, and central and eastern Europe are historically iodine deficient(Reference Zimmermann1). Iodine deficiency in livestock and humans in these areas will persist until iodine enters the food chain through fortification of iodine to foods (e.g. iodisation of salt) or dietary diversification introduces foods produced outside the iodine-deficient area(2).
Dietary requirements, intake and metabolism
The WHO has established recommended nutrient intakes for iodine (Table 1)(2). The US Food and Nutrition Board of the National Academy of Sciences has set an adequate intake for iodine in infancy and a RDA for children, adults and pregnant and lactating women(3) (Table 1). Most foods and beverages have low native iodine content. In general, commonly consumed foods provide 3–80 μg per serving(4,Reference Haldimann, Alt and Blanc5) . In the UK, rich dietary sources of iodine are dairy products and seafood, with 200 ml of cow's milk providing 50–100 μg (depending on the season, with higher values in winter), 120 g of cod providing 230 μg, 170 g of shrimp providing 160 μg and one egg (50 g) providing 25 μg iodine(Reference Haldimann, Alt and Blanc5). Foods of marine origin have higher iodine content as marine plants and animals tend to concentrate iodine from seawater. Major dietary sources of iodine in Europe, North America and Australia are bread products made with iodised salt and milk(Reference Pearce, Pino and He6). In Switzerland, based on direct food analysis, mean intake of dietary iodine is about 140 μg/d, mainly from bread and dairy products(Reference Haldimann, Alt and Blanc5). Much of the iodine content of dairy products is adventitious, and enters the milk during processing, for example, as iodine-containing disinfectants. In many countries, use of iodised salt in households for cooking and at the table is an important source of iodine. Iodine is often included in multivitamin-mineral supplements. Based on data from the US Third National Health and Nutrition Examination Survey, 15 % of non-pregnant women and 12 % of men took a supplement that contained iodine, and the median intake of iodine from supplements was about 140 μg/d for adults(3). Goitrogens are dietary substances that may interfere with thyroid metabolism and can exacerbate the effect of iodine deficiency. For example, vegetables of the Brassica family (i.e. cabbage, cauliflower, broccoli) contain glucosinolates, which are goitrogens. However, most goitrogens do not have major effects unless they are consumed at very high levels and iodine deficiency is also present(Reference Zimmermann1).
Table 1. Recommendations for iodine intake (μg/d) by age or population group
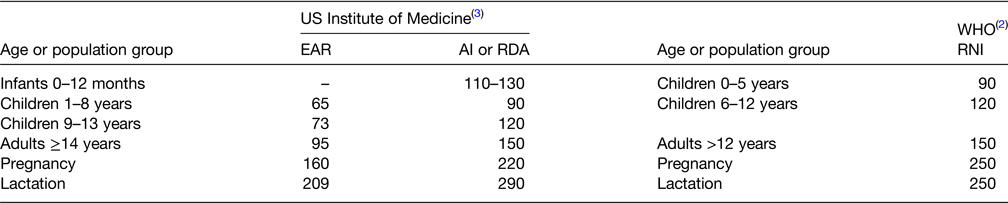
AI, adequate intake; EAR, estimated average requirement; RDA, recommended daily allowance; RNI, recommended nutrient intake.
Dietary iodide is rapidly absorbed in the stomach and duodenum. In healthy adults, the absorption of iodide is generally >90 %(Reference Zimmermann1). Iodine is cleared from the bloodstream mainly by the thyroid and kidney. Although renal iodine clearance is fairly constant, thyroid clearance varies with iodine intake. If dietary iodine intake is adequate, ≤10 % of absorbed iodine is taken up by the thyroid. In contrast, in chronic iodine deficiency, this fraction can exceed 80 %(Reference Zimmermann1). During lactation, the mammary gland concentrates iodine from the bloodstream and secretes it into breast milk. The body of a healthy adult contains up to 20 mg of iodine, of which 70–80 % is in the thyroid(Reference Zimmermann1). In iodine-sufficient areas, to balance losses and maintain thyroid hormone synthesis, the adult thyroid traps about 60 μg of iodine/d, either from dietary iodine or from iodine released during thyroid hormone turnover. Thyrocytes produce and secrete the two thyroid hormones, thyroxine (T4) (the major form) and triiodothyronine (T3). In target tissues, including liver, kidney, heart, muscle, pituitary and the developing brain, T4 is converted to T3. T3 is the main physiologically active form of thyroid hormone and regulates a variety of physiologic processes, including reproductive function, growth and development, as well as the BMR(Reference Zimmermann1). During pregnancy, thyroid hormone crosses the placenta to the fetus early in the first trimester, before the fetal thyroid is functioning(Reference Zimmermann7). In the developing brain, it influences cell growth and migration(Reference Morreale de Escobar, Obregon and Escobar del Rey8). More than 90 % of ingested iodine is ultimately excreted in the urine, with only a small amount appearing in the faeces(Reference Zimmermann1).
Iodine deficiency disorders
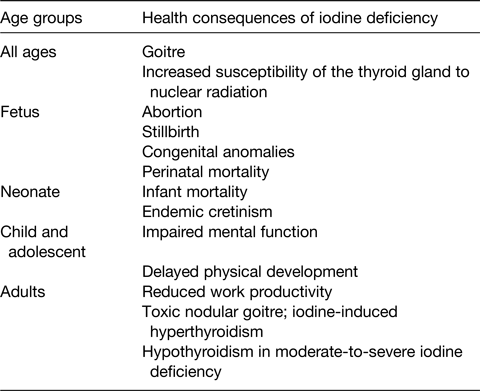
Iodine deficiency has multiple detrimental effects on growth and development in human subjects. These are collectively termed the iodine deficiency disorders (IDD)(2) (Table 2), and, until recently, were one of the most important and common human diseases. They all result from inadequate thyroid hormone production due to lack of sufficient iodine. Thyroid enlargement (goitre) is the classic sign of iodine deficiency. It is a physiologic adaptation to chronic iodine deficiency. As dietary iodine intake falls, secretion of pituitary thyroid-stimulating hormone increases to maximise uptake of circulating iodine by the thyroid, and thyroid-stimulating hormone stimulates thyroid hypertrophy and hyperplasia(Reference Zimmermann1). Large goitres can obstruct the trachea and oesophagus and may damage the recurrent laryngeal nerves. Goitre is the most visible effect of iodine deficiency, but the most serious adverse effect is damage to reproduction, and impaired fetal and child development. Severe iodine deficiency during gestation increases risk for stillbirths, abortions and congenital abnormalities(Reference Zimmermann1). The fetal brain is particularly vulnerable to iodine deficiency, as thyroid hormones are required for neuronal migration and myelination in the rapidly developing central nervous system(Reference Morreale de Escobar, Obregon and Escobar del Rey8). The most severe form of neurological damage from fetal hypothyroidism is termed cretinism. It is characterised by gross mental retardation along with varying degrees of short stature, deaf mutism and spasticity(Reference Zimmermann1). Up to 10 % of populations with severe iodine deficiency may be cretinous. Non-cretinous offspring of severely iodine-deficient mothers are at high risk for impaired cognitive development. Meta-analysis studies concluded that moderate-to-severe iodine deficiency in populations reduces mean IQ scores by up to 13⋅5 points(Reference Bleichrodt, Born and Stanbury9). Before widespread salt iodisation, iodine deficiency was one of the most common causes of preventable mental retardation worldwide(2). Fortunately, iodised salt programmes in most countries have controlled severe iodine deficiency and eradicated cretinism. However, mild iodine deficiency still affects many pregnant women(Reference Zimmermann, Gizak and Abbott10), and it remains uncertain whether mild deficiency during pregnancy can impair cognitive development of the offspring. In randomised controlled trials in school-aged children in areas of mild-to-moderate iodine deficiency, iodine supplementation improved cognition(Reference Taylor, Okosieme and Dayan11). Iodine intake is also a major determinant of the pattern of thyroid disorders in adults(Reference Zimmermann and Boelaert12). In areas of mild-to-moderate deficiency, there is an increased risk of toxic goitre and hyperthyroidism, especially in older women(Reference Zimmermann and Boelaert12). Overall, iodine deficiency triggers adverse health effects at all ages, decreasing educability and productivity, and impairing social and economic development(2).
Table 2. Iodine deficiency disorders, by age group(2,Reference Zimmermann and Boelaert12)
Assessment of iodine status
Two methods are commonly used to assess iodine nutrition: the goitre rate and the urinary iodine concentration (UIC)(2). UIC is a sensitive indicator of recent iodine intake (days to weeks), whereas changes in the goitre rate reflect long-term iodine nutrition (months to years)(Reference Zimmermann and Andersson13). Goitre surveys are usually done in school-aged children. By palpation, a thyroid is considered goitrous when each lateral lobe has a volume greater than the terminal phalanx of the thumbs of the subject being examined(2). In areas of mild iodine deficiency, where goitres are small, measurement of thyroid size by ultrasonography is a more objective and precise method and is preferable to palpation(2). Goitre is classified according to international reference criteria for thyroid size in iodine-sufficient children. The total goitre rate is used to define severity using the following criteria: <5 %, iodine sufficiency; 5⋅0–19⋅9 %, mild deficiency; 20⋅0–29⋅9 %, moderate deficiency; and >30 %, severe deficiency(2).
Because in healthy individuals, >90 % of ingested iodine is excreted in the urine, UIC is a good indicator of recent iodine intake(2,Reference Zimmermann and Andersson13) . UIC can be expressed as a concentration (μg/l), in relation to urinary creatinine excretion (μg iodine/g creatinine), or as 24 h urinary excretion (μg/d). For population surveys or cross-sectional studies, it is recommended that UIC be measured in spot urine specimens from a representative sample of the target group, and expressed as the median, in μg/l. Variations in hydration among individuals generally even out in large surveys, so that the median UIC in spot samples correlates well with that from 24 h collections(Reference Zimmermann and Andersson13). It is a common mistake to assume that all subjects with a spot UIC <100 μg/l in a survey are iodine deficient, because even in iodine-sufficient regions, individual spot UIC concentrations are highly variable from day-to-day. Therefore, a spot UIC should not be used to classify individual iodine status. Rather, the median UIC should be used to classify a population's iodine status(2) (Table 3). Recent data suggest the WHO median UIC categories (Table 3) of ‘adequate’ and ‘more than adequate’ iodine intake recommended in children can be combined into a single category (100–299 μg/l) to denote adequate iodine nutrition(Reference Zimmermann, Aeberli and Andersson14). Pregnant and lactating women have high iodine requirements and are at higher risk of deficient intakes. Targeted monitoring of iodine status in these vulnerable groups is important, particularly in countries where the general population has borderline adequate iodine intakes or mild iodine deficiency(Reference Zimmermann, Gizak and Abbott10). Iodine status in lactating women should be monitored by measuring the UIC and iodine concentration in breast milk. Although the median UIC does not provide direct information on thyroid function, a low value suggests that a population is at higher risk of developing thyroid disorders(Reference Zimmermann and Boelaert12).
Table 3. Epidemiological criteria from the WHO(2,18) for assessment of iodine nutrition in a population based on median urinary iodine concentrations
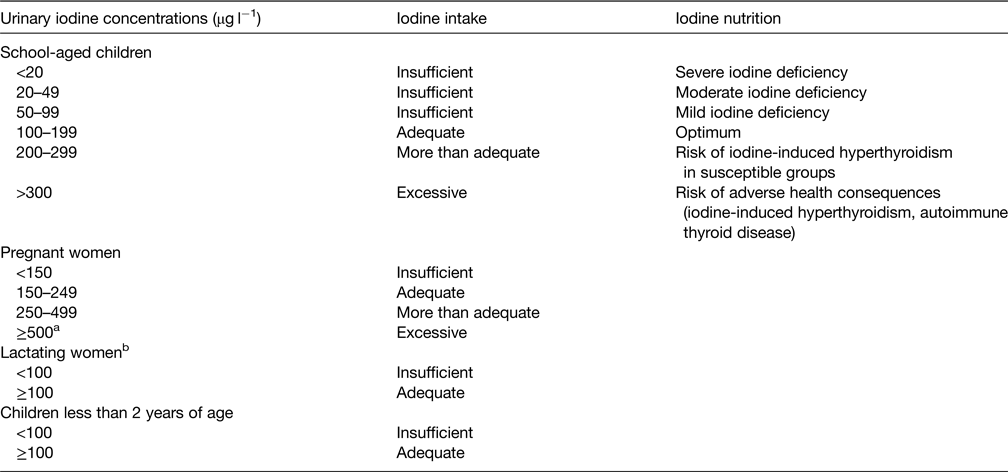
a The term excessive means in excess of the amount needed to prevent and control iodine deficiency.
b In lactating women, the numbers for median urinary iodine are lower than the iodine requirements, because of the iodine excreted in breast milk.
Prophylaxis and treatment of iodine deficiency
Two methods are commonly used to correct iodine deficiency: iodine supplements and iodised salt(2). In nearly all regions affected by iodine deficiency, the most effective way to control iodine deficiency at the population level is through salt iodisation. All salt for human consumption, including salt used in the food industry, should be continuously iodised. Iodine can be added to salt in the form of potassium iodide (KI) or potassium iodate (KIO3) and is usually added at a level of 15–45 μg iodine/g, depending on local salt intake(2). In many high- and middle-income countries, because >80 % of salt consumption is from purchased processed foods, if only household salt is iodised it may not supply adequate iodine(Reference Bhat, Marklund and Henry15,Reference Spohrer, Garrett and Timmer16) . Thus, it is critical that the food industry uses iodised salt. The current push to reduce salt consumption to prevent chronic diseases and the policy of salt iodisation to eliminate iodine deficiency do not conflict(Reference Campbell, Dary and Cappuccio17): iodisation methods can fortify salt to provide recommended iodine intakes even if per capita salt intakes are reduced to <5 g/d.
In some regions, iodisation of salt may not be practical for control of iodine deficiency, at least in the short term. This may occur in remote areas where salt supply is limited or where there are numerous small-scale salt producers(2). In these areas, other options for correction of iodine deficiency should be considered. Long-lasting supplements of iodised oil are recommended for populations with moderate-to-severe iodine deficiency that do not have access to iodised salt; these can be targeted towards pregnant and non-pregnant women, and young children. The recommended dose is 400 mg of iodine per year for women and 200 mg of iodine per year for children 7–24 months of age(18). Iodine can also be given as potassium iodide or iodate as drops or tablets. Many expert groups recommend oral iodine supplements (about 150 μg/d) for pregnant and lactating women residing in areas of mild-to-moderate iodine deficiency, whose increased iodine requirements may not be completely covered by iodised salt(Reference Zimmermann, Gizak and Abbott10).
Global control of iodine deficiency: a public health triumph
Only a few countries, including Switzerland, the Scandinavian countries, Australia, the US and Canada, were completely iodine sufficient before 1990(Reference Zimmermann1). They had adequate intakes due to iodised salt programmes and adventitious iodine added during processing of dairy foods. Since then, widespread introduction of iodised salt has produced dramatic reductions in iodine deficiency. WHO's first estimate of the global prevalence of goitre in 1960 suggested that 20–60 % of the world's population was affected, with most of the burden in low- and middle-income countries(Reference Kelly and Snedden19). Subsequently, the International Child Development Steering Group highlighted iodine deficiency as one of four key global risk factors for impaired child development where the need for intervention was urgent(Reference Walker, Wachs and Gardner20). This spurred a global effort to eliminate iodine deficiency led by a coalition of international organisations, including UNICEF, WHO, the International Council for the Control of IDD, the Iodine Global Network, the Micronutrient Initiative and the Global Alliance for Improved Nutrition, working closely with national IDD control committees and the salt industry; this informal partnership was established after the World Summit for Children in 1990. Major funders of this effort have included Kiwanis International, the United States Agency for International Development, the Bill & Melinda Gates Foundation, and many country foreign aid programmes.
In 2020, 124 countries had legislation for mandatory salt iodisation and twenty-one had legislation allowing voluntary salt iodisation(21). The reach of current iodised salt programmes is remarkable. UNICEF estimates that, based on data collected during the period 2013–2018, 88 % of the global population was using iodised salt in 2018(22). South Asia and East Asia and the Pacific had the highest household coverage with iodised salt at 89 and 92 %, respectively. Western and Central Africa had the lowest coverage with iodised salt, but still over three in four households in the region had access to iodised salt(22).
The Iodine Global Network compiles data from UIC studies conducted throughout the world and continually monitors global iodine status(23). The Iodine Global Network Scorecard and Map presents the most recent UIC data for 194 WHO Member States, plus Liechtenstein and Palestine (Fig. 1). In population monitoring of iodine status using UIC, WHO recommends that school-age children (SAC) serve as a proxy for the general population(2). Therefore, in the Iodine Global Network Scorecard, preference is given to studies carried out in SAC. Data are selected for each country in the following order of priority(23): data from the most recent known nationally representative survey carried out between 2005 and 2020 in (a) SAC, (b) SAC and adolescents, (c) adolescents, (d) women of reproductive age and (e) other adults (excluding pregnant or lactating women). In the absence of recent national surveys, subnational data are used in the same order of priority. In the Scorecard, adequate iodine intake in SAC, women of reproductive age and other adults is defined based on a median UIC in the range 100–299 μg/l(Reference Zimmermann, Aeberli and Andersson14).
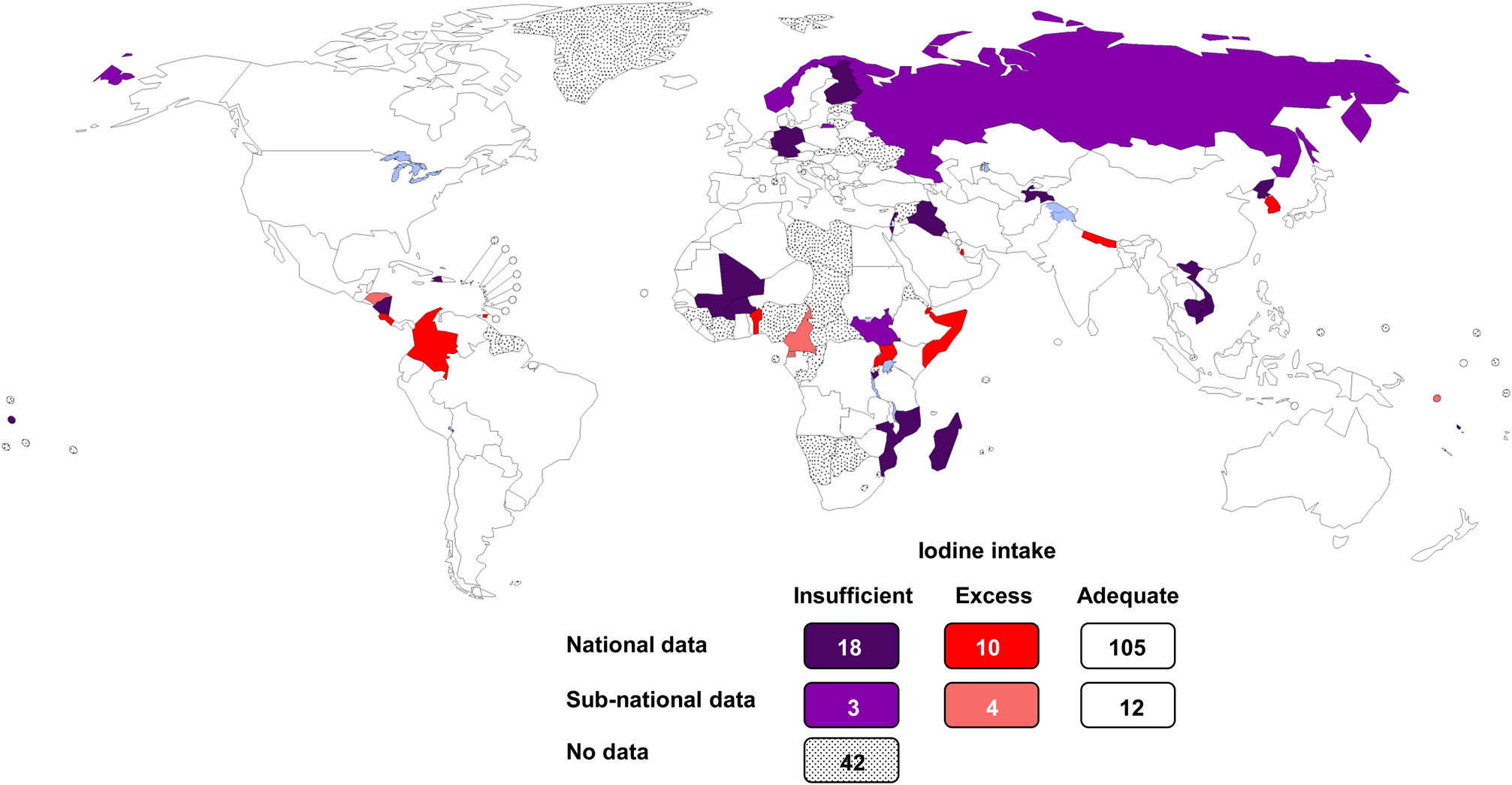
Fig. 1. Estimated iodine nutrition in 194 WHO Member States in 2020 based on national median UIC in school-age children obtained from studies conducted between 2005 and 2020(23). UIC, urinary iodine concentration.
Cross-sectional UIC studies have been conducted in 152 out of 194 countries in the past 15 years: in 133 countries, the studies were nationally representative(23). The iodine intake in the general population is adequate in 117 countries. The number of countries with adequate iodine intake has nearly doubled over the past 20 years from 67 in 2003(Reference Andersson, Takkouche and Egli24), to 105 in 2011(Reference Andersson, Karumbunathan and Zimmermann25) and to 118 in 2020(23), reflecting successful implementation of salt iodisation worldwide. Recent data show that mandatory salt iodisation at 25 ppm ensures adequate iodine intake in all population groups, including pregnant and lactating women, who have increased requirements(Reference Dold, Zimmermann and Jukic26). Bouillon cubes containing iodised salt are additional important dietary iodine sources and increasingly contribute to iodine intake in low- and middle-income countries(Reference Abizari, Dold and Kupka27).
In 2020, globally, twenty-one countries still have insufficient iodine in their diets (Fig. 1)(23). Iodised salt is generally available in these countries, but the coverage is poor or incomplete. Madagascar has the lowest iodine intake, because the mandatory salt iodisation programme fell apart due to political instability and is only now being rebuilt. In Cambodia, the production of iodised salt declined when donors stopped supplying KIO3 and the amount of iodine in fortified salt decreased. Weakening of mandatory legislation in Vietnam allowed introduction of non-iodised salt and iodine deficiency has recurred. Several countries (e.g. Sudan, Burkina Faso and Russia) have patchy national coverage with iodised salt and large regional variations in iodine status. In Haiti and Iraq, natural disasters and war, respectively, disrupted implementation and monitoring of salt production and the distribution chain. Notably, the iodine intake is also inadequate in several European countries with strong health systems (e.g. Norway, Germany and Finland); the major challenge in these countries is the low use of iodised salt in the production of processed foods, which contribute most dietary salt.
Health and economic benefits
Salt iodisation remains the most cost-effective way of delivering iodine and of improving cognition in iodine-deficient populations(Reference Engle, Black and Behrman28). Worldwide, the annual costs of salt iodisation are estimated at US$0⋅02–0⋅05 per child covered, and the costs per child death averted are US$1000 and per disability-adjusted life year gained are US$34–36(Reference Caulfield, Richard, Rivera, Dean, Jamison, Breman, Measham, Alleyne, Claeson, Evans, Jha, Mills and Musgrove29). Prior to widespread salt iodisation, the annual potential losses attributable to iodine deficiency in the developing world have been estimated to be US$35⋅7 billion as compared with an estimated US$0⋅5 billion annual cost for salt iodisation, i.e. a 70:1 benefit:cost ratio(Reference Horton30). A recent analysis using a regression model estimated the potential health and economic benefits realised between 1993 and 2019 by the global programme to reduce iodine deficiency and goitre(Reference Gorstein, Bagriansky and Pearce31). Based on this approach, the global prevalence of clinical IDD (as assessed by the total goitre rate) fell from 13⋅1 to 3⋅2 %. USI has significantly reduced the number of newborns affected by IDD, with 20⋅5 million cases prevented annually. The resulting improvement in cognitive development and future earnings suggest a potential global economic benefit of nearly $33 billion(Reference Gorstein, Bagriansky and Pearce31).
Conclusions and remaining challenges
Despite rapid progress in salt iodisation worldwide, there remain concerns. UNICEF states there are insufficient recent data available to generate estimates of iodised salt coverage for Central Asia, Latin America and the Caribbean, and Middle East and North Africa(22). The UNICEF salt iodisation data refer to salt with any iodine (>0 ppm) but the ultimate goal of iodised salt programmes is to achieve ‘sufficiently’ iodised salt, that is, in the range of 15–40 ppm(32). According to the Global Fortification Data Exchange, only 89 of the 145 countries with legislation on salt iodisation have gathered recent data on coverage(21). The regression model of iodised salt impact based on total goitre rates suggests that 4⋅8 million newborns were still affected by IDD in 2019, who will experience life-long productivity losses totalling a net present value of $12⋅5 billion(Reference Gorstein, Bagriansky and Pearce31). Thus, salt iodisation programmes need to be expanded, particularly in sub-Sahara Africa and Asia, and monitoring of these programmes needs to be strengthened. Also, in typical Western-style diets, processed foods such as bread, dairy products and processed meats are major salt sources, but the salt used in these foods is often not iodised. The food industry should be encouraged to use iodised salt, as its addition does not change the colour or flavour of foods.
Although most people are remarkably tolerant to high dietary intakes of iodine, iodine intake far above requirements is unnecessary and should be avoided. Excessive intake of iodine in previously iodine-deficient areas may precipitate a transient increase in hyperthyroidism(Reference Zimmermann and Boelaert12). Globally, thirteen countries have documented excessive iodine in their diets, as defined by a median UIC >300 μg/l (Fig. 1)(23). Excess iodine intakes in populations can result from diets that are naturally high in iodine and/or groundwater (e.g. South Korea, Somalia, Djibouti). Iodine excess can also occur when the level of iodine added to salt is too high considering per capita salt intakes (e.g. Cameroon, Honduras, Columbia). The US Food and Nutrition Board has set tolerable upper intake levels for iodine(3). The tolerable upper intake level is the highest level of daily intake that is likely to pose no risk of adverse health effects in almost all individuals. The tolerable upper intake level is 200 μg/d for ages 1–3 years, 300 μg/d for ages 4–8 years, 600 μg/d for ages 9–13 years, 900 μg/d for ages 14–18 years and 1100 μg/d thereafter(3). However, individuals with autoimmune thyroid disease or chronic iodine deficiency may respond adversely to intakes lower than these(Reference Zimmermann and Boelaert12).
In summary, the global improvements in iodine status over the past 30 years have resulted in major health and economic benefits, mainly in low- and middle-income countries. Efforts should now focus on sustaining this achievement(Reference Pretell, Pearce and Moreno33) and expanding salt iodisation to reach the handful of countries remaining iodine deficient.
Acknowledgements
Maria Andersson (Children's Hospital Zurich) provided advice on the manuscript.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of preparation of the paper.







