Suboptimal diets are responsible for the highest rates of morbidity and mortality globally, with recent data indicating that improvement in dietary intake could potentially prevent one in every five deaths(1). Concomitant with this, the burden of some diet-related diseases, especially diabetes, continues to increase(2). This scenario has led to questions regarding the effectiveness of the current dietary guidelines for populations in improving health(Reference Ordovas, Ferguson and Tai3,Reference Adams, Anthony and Carvajal4) . In recent years, the importance of metabolic interindividual variability emerged as a key factor that drives differential responses to food. Indeed, CVs between 59 and 103 % were reported for postprandial TAG, glucose and insulin in response to identical meals with the contribution of clinical, microbiome and lifestyle factors differing greatly among outcomes(Reference Berry, Valdes and Drew5). To account for interindividual variability, a variety of approaches providing personalised nutrition were developed and achieved positive results(Reference Zeevi, Korem and Zmora6–Reference Horne, Gilliland and O'Connor12). For example, an algorithm to personalise diets that used clinical and microbiome factors resulted in improvement in glycaemic control compared to a Mediterranean diet(Reference Ben-Yacov, Godneva and Rein7). The Food4Me study reported that personalised advice based on dietary intake data only or in combination with phenotype and genotype improved dietary quality compared to generic advice(Reference Celis-Morales, Livingstone and Marsaux9). Genetically tailored advice applied to weight management resulted in greater reductions in total fat intake and better long-term adherence to total fat and saturated fat guidelines compared to generic advice(Reference Horne, Gilliland and O'Connor12). Across the majority of these studies, complex data were employed to personalise nutrition advice. While this is effective at an individual level, there is an urgent need to develop more personalised strategies for the prevention of diet-related diseases that are feasible and affordable for implementation in populations.
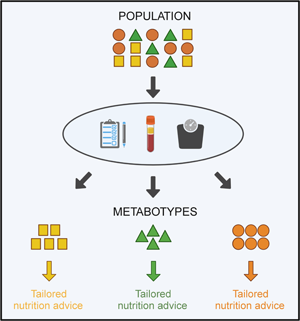
In pursuit of this goal, metabotyping is an approach for the identification of individuals that could benefit from tailored nutrition advice. Metabotyping uses metabolic parameters to group individuals into subgroups with similar metabolic profiles(Reference Riedl, Gieger and Hauner13–Reference Brennan15). For the purpose of this review, we will refer to subgroups of individuals with similar metabolic characteristics as metabotypes, while acknowledging that other terms such as data-driven clusters, subphenotypes and metabolic subgroups are also used in the literature. Metabotypes are strongly anchored on underlying physiological mechanisms and reflect the individual interactions between internal and external exposures and genes(Reference Kaput14,Reference Brennan15) . In human nutrition research, metabotypes emerged by demonstrating that subgroups of individuals with similar metabolic profiles have differential responses to dietary challenges and interventions(Reference Krishnan, Newman and Hembrooke16–Reference Fiamoncini, Rundle and Gibbons19). Subsequently, metabotypes were associated with differential prevalence and incidence of diseases thus suggesting that they could be used to tailor prevention strategies(Reference Riedl, Hillesheim and Wawro20–Reference Ventura, Loken and Birch22). These studies provided the evidence base for the development of frameworks to deliver personalised nutrition using group characteristics. Currently, the major focus of the application of metabotyping is the identification of homogeneous and clinically relevant subgroups for which optimal interventions could be designed to positively impact health outcomes (Fig. 1). This review will discuss the evidence supporting the use of metabotyping to tailor nutrition advice drawing on the broader literature including emerging studies in the diabetes field.

Fig. 1. Overview of the evidence development on metabotyping for the delivery of tailored nutrition advice.
Ability of metabotyping to identify differential response to dietary challenges and interventions
Metabotyping can be applied to nutrition studies to examine the responses of groups to dietary challenges and interventions. These studies constitute an important step to understanding the variability existing among individuals and the rationale of using metabotyping to obtain information to tailor nutrition advice based on group characteristics.
For example, overweight and obese women were clustered based on their glucose, insulin and leptin responses to meals differing in the glycaemic index to investigate patterns of subclinical glycaemic disruptions(Reference Krishnan, Newman and Hembrooke16). While the most populated metabotype presented little deviation from the expected responses to the dietary challenges, the two minor metabotypes were one suggestive of hyperleptinaemia with high leptin and glucose and the other suggestive of sub-clinical insulin resistance with lower postprandial leptin and higher insulin and glucose responses. A similar approach clustered overweight Asian women into three metabotypes based on their glucose, insulin and TAG responses to two high-protein meal challenges high in fructose or glucose(Reference Camps, Koh and Wang23). In addition to a metabotype with average responses, the same stimulus revealed other two metabotypes with adverse metabolic responses. The group susceptible to visceral fat and liver fat accumulation presented the highest TAG response and waist-to-hip ratio and worst lipid profile whereas the group vulnerable to prediabetes presented the highest glucose response, fasting glucose, BMI, fat percentage and hip circumference. Using only blood glucose response curves to an oral glucose tolerance test to cluster healthy individuals, another study found an ‘at-risk’ metabotype among four identified metabotypes(Reference Morris, O'Grada and Ryan18). The at-risk metabotype had the most adverse metabolic profile with reduced β-cell function, impaired insulin and C-peptide responses to the oral glucose tolerance test and impaired glucose, insulin, C-peptide and TAG responses to an additional oral lipid tolerance test. These studies demonstrate that metabotyping may be useful for detecting subclinical metabolic dysfunctions and could contribute to developing and optimising personalised nutrition interventions.
Metabotypes have also been defined using fasting variables and their patterns of metabolic responses investigated using dietary challenges. Using a series of blood metabolites from postmenopausal women a metabotype suggestive of insulin resistance was identified(Reference Moazzami, Shrestha and Morrison24). Compared to a healthier metabotype, the insulin resistance group initially characterised by higher levels of fasting leucine and isoleucine and lower levels of fasting sphingomyelins and phosphatidylcholines had the highest insulin despite similar glucose concentrations in response to challenges with different types of bread. In a study designed to investigate the vascular effects of a high-saturated fat meal and a mixed Mediterranean-type meal individuals were clustered based on age, BMI and lipid parameters(Reference Lacroix, Des Rosiers and Gayda25). The high-saturated fat meal produced endothelial dysfunction only in the unhealthy metabotype characterised by higher BMI, insulin resistance, total cholesterol and TAG. In both groups, the mixed Mediterranean-type meal did not significantly impact postprandial endothelial function suggesting that the unhealthy metabotype could benefit even more from a Mediterranean diet.
Retrospective application of metabotyping to interventions revealed a responsive metabotype in a number of studies. In a 12-week weight loss intervention, positive changes to a mixed meal tolerance test were evident only after the classification of individuals into metabotypes using plasma levels of metabolites (markers of lipolysis, fatty acid β-oxidation and ketogenesis)(Reference Fiamoncini, Rundle and Gibbons19). The responsive metabotype, which was considered prediabetic with a modestly impaired insulin action at baseline, presented reductions in postprandial glycaemia, adipose tissue depots and plasma levels of amino acids and acylcarnitine becoming more similar to the individuals in the non-responsive group. Similarly, improvements in markers of metabolic syndrome were observed with a 4-week vitamin D supplementation only in a responsive metabotype identified among five using thirteen biochemical markers at baseline(Reference O'Sullivan, Gibney and Connor17). The metabotype characterised by low concentrations of vitamin D and higher concentrations of adipokines at baseline presented an inverse relationship between the change in serum vitamin D and glucose and significant reductions in C-reactive protein, insulin and homoeostatic model assessment for insulin resistance. In the 10-year RCT Look AHEAD, post-hoc analysis defined metabotypes for patients with diabetes using four clinical variables (age at diabetes diagnosis, BMI, waist circumference and glycated Hb) and a poor glucose control metabotype that received an intensive lifestyle (dietary and exercise) intervention (LI) was associated with an increased risk of CVD events compared to the same metabotype that received a diabetes support and education intervention(Reference Bancks, Chen and Balasubramanyam26). In the three remaining metabotypes, the risk of CVD events was similar between study groups, but the intensive LI improved multiple CVD risk factors. These findings demonstrate that metabotyping may play a role in determining the appropriate intervention for subgroups of individuals.
In a German cohort, BMI and thirty-two biochemical markers were used to identify three metabotypes with different levels of metabolic impairment and incidence of diet-related diseases(Reference Riedl, Wawro and Gieger21). The metabotype with the most unfavourable biomarker profile and the highest BMI and prevalence of cardiometabolic diseases at baseline also presented the highest incidence of hypertension, type 2 diabetes, hyperuricaemia/gout, dyslipidaemia and all cardiometabolic diseases in a 7-year follow-up. The work supports the role of metabotyping as a robust tool for risk stratification and consequently for tailoring prevention strategies. In the same cohort, three metabotypes were defined with a reduced set of variables (HDL-C, non-HDL-C, uric acid, fasting glucose and BMI) and investigated for their responses to an oral glucose tolerance test and a 12-week fibre intervention(Reference Dahal, Wawro and Meisinger27). Compared to the healthy metabotype, participants in the intermediate and unfavourable metabotypes presented impaired glucose responses to the oral glucose tolerance test with significantly higher postprandial glucose concentrations. Although the fibre intervention did not significantly change metabolic parameters across metabotypes, the group with an unfavourable profile had the greatest reductions in insulin, cholesterol parameters (total cholesterol, LDL-C and non-HDL-C) and blood pressure. These metabotypes were also applied to investigate their effect on associations between diet and DNA methylation(Reference Hellbach, Baumeister and Wilson28). While only a few significant associations were observed in the total cohort, many were observed when the analyses were stratified by metabotypes; most of them between methylation sites and the intake of cruciferous, cheese, whole grain products, margarine, eggs and total meat. This highlights the importance of including information on the metabolic profile of participants in diet–epigenome association studies.
In summary, there is compelling evidence that metabotyping can stratify individuals into homogeneous groups with differential responses to dietary challenges and interventions. This provides a solid base for further investigation of metabotypes as a means to tailor nutrition advice at a group level.
Potential of metabotyping to personalise healthcare illustrated by diabetes research
The investigation of metabotypes to provide personalised healthcare extends now to a variety of medical fields(Reference Dickinson, Zaidman and Giangrande29–Reference Richette, Clerson and Périssin34). There is growing interest and important advances especially in applying metabotypes to unravel the complexity of prediabetes and diabetes by defining subgroups that display patterns of phenotypes and complications. These health conditions are currently recognised as highly heterogeneous in their pathophysiological mechanisms which result in differences in the patients' clinical presentations already at diagnosis(Reference Zaharia, Strassburger and Strom35,Reference Chan, Lim and Wareham36) . In this context, the identification of metabotypes with the ability to predict disease prognosis illustrates the potential of this tool for preventative healthcare strategies (Table 1).
Table 1. Examples of studies identifying metabotypes in prediabetes and diabetes populations

GADA, glutamate decarboxylase antibodies; HbA1c, glycated Hb; HOMA-IR, homoeostatic model assessment for insulin resistance; HOMA-B, homoeostatic model assessment of β-cell function; SRD, Scania Diabetes Registry; DIREVA, Diabetes Registry Vaasa; ANDIU, All New Diabetics in Uppsala; PRS, polygenic risk score; T2D, type 2 diabetes; N/A, not available; IR, insulin resistance; CKD, chronic kidney disease; GLP, glycerophospholipids; SM, sphingomyelins; CER, ceramides; LPC, lysophosphatidylcholines; TC, total cholesterol; MMTT, mixed meal tolerance test; ALT, alanine transaminase; AST, aspartate aminotransferase; ISI, insulin sensitivity index; OGTT, oral glucose tolerance test; HDL-C, HDL cholesterol; SNP, single-nucleotide polymorphisms; WC, waist circumference; HC, hip circumference; SIDD, severe insulin-deficient diabetes; SIRD, severe insulin-resistant diabetes; MOD, mild obesity-related diabetes; MARD, mild age-related diabetes; SAID, severe autoimmune diabetes.
In patients with diabetes from the Swedish Cohort ANDIS, metabotypes identified at disease diagnosis were associated with the risk of developing diabetic complications(Reference Ahlqvist, Storm and Käräjämäki37). Six readily available clinical variables were used to define five clusters related to autoimmune diabetes, insulin deficiency, insulin resistance, obesity and age. In a 4-year follow-up, the clusters differed in disease progression and complications. Of note, the insulin resistance cluster exhibited the highest risk of diabetic kidney disease and the insulin deficiency cluster exhibited the highest risk of retinopathy. These clusters have been extensively replicated in several cohorts of diverse ethnicities(Reference Zaharia, Strassburger and Strom35,Reference Zou, Zhou and Zhu38–Reference Tanabe, Saito and Kudo41) and associated with genetics(Reference Mansour Aly, Dwivedi and Prasad42) and response to medical treatment(Reference Raverdy, Cohen and Caiazzo43). Further characterisation of these clusters could provide useful information to design tailored nutrition interventions to assist the management of diabetes and its complications.
Although the ANDIS clusters have been replicated in several populations, a study in a Singaporean cohort highlights the impact of certain ethnicities on disease profile and progression and the importance of testing the metabotyping model in the target population before considering its application(Reference Wang, Liu and Gurung44). Using the same ANDIS method and markers (except for glutamate decarboxylase antibody that determined autoimmune diabetes), a total of three clusters were reported. The cluster characterised by obesity and insulin resistance contained the highest proportion of patients (45 %) indicating that prevention and treatment of these conditions are the major factors to slow down the increasing prevalence of diabetes in this Singaporean population. However, the most notable difference was the absence of a cluster exclusively characterised by insulin deficiency; instead, this trait was diffused into two clusters related one to insulin resistance and the other to age. Patients in the cluster with insulin insufficiency and resistance, despite being more than 10 years younger and having similar diabetes duration to the other clusters, presented the highest risk for chronic kidney disease and the same risks of major CVD events and all-cause mortality suggesting a high-risk cluster that should be closely followed up.
Recently, using a soft-clustering approach on thirty-two anthropometric, clinical and biochemical variables four main clusters were identified in patients newly diagnosed with diabetes(Reference Wesolowska-Andersen, Brorsson and Bizzotto45). This approach classifies individuals with a top score in one specific cluster but allows those with lower scores to be members of multiple clusters (mixed phenotype). Analysis of a 36-month follow-up revealed that patients from a cluster characterised by obesity, dyslipidaemia, insulin resistance and β-cell dysfunction presented the fastest disease progression and the highest demand for anti-diabetic treatment. Mixed phenotype patients with this cluster as a primary or secondary cluster showed a trend towards faster progression except when combined with a cluster characterised as lean and insulin deficient. This study demonstrates that multiple phenotypes modulate disease progression and understanding the interaction between them may help to stratify patients and guide targeted interventions.
In individuals with prediabetes, metabotyping was applied with the concept of developing preventative measures(Reference Wagner, Heni and Tabák46). In a cohort of German individuals with increased risk for type 2 diabetes, six clusters were defined and differed in the progression to the disease. Although three clusters were similarly characterised by increased glycaemia, only two clusters were associated with imminent risk of diabetes in a 4-year follow-up: one with the highest genetic risk for diabetes and the lowest insulin secretion and another with characteristics of well-established metabolic syndrome. In contrast, the third cluster presented a low incidence of diabetes but severe insulin resistance had an increased risk of kidney disease and all-cause mortality. Based on the characteristics of the at-risk clusters, the authors suggested that LIs tailored to each cluster could prevent the progression to diabetes. Importantly, the clusters previously defined using a series of complex variables, including clinical biochemistry, body and organ fat content and genetics, were replicated in a British cohort using simpler proxy variables and worked impressively well, suggesting that clinical variables may be sufficient to predict the same health outcomes. This study provides an elegant example of the opportunities that metabotyping offers for screening and the development of interventions to prevent diabetes in at-risk individuals.
In addition to the key studies abovementioned, several others have been published using metabotypes in prediabetes and diabetes patients(Reference Herder and Roden47–Reference Gouda, Zheng and Peters49). Overall, there is an agreement that this is a promising approach to refining the current disease classifications beyond levels of glucose and insulin. Altogether, these studies demonstrate that metabotyping is a stepping stone towards providing personalised healthcare which could be further developed to include nutrition advice for preventative measures.
Metabotyping as a tool to deliver personalised nutrition
Developing frameworks to deliver targeted or personalised nutrition and testing their effectiveness in randomised controlled trials (RCTs) is an essential step to translate the metabolic information provided by metabotypes into clinical application. However, evidence in this field is in its infancy with only a few protocols and studies published which are diverse in the objectives and population investigated (Table 2).
Table 2. Studies and protocols investigating the delivery of personalised/targeted nutrition and lifestyle interventions using metabotypes

RCT, randomised controlled trial; TC, total cholesterol; HDL-C, HDL cholesterol; PG, personalised group; WC, waist circumference; BP, blood pressure; CG, control group; N/A, not available; SNPs, single-nucleotide polymorphisms; FM, fat mass; DXA, dual-energy X-ray absorptiometry; BW, body weight; OxLDL, oxidised LDL; HOMA-IR, homoeostatic model assessment of insulin resistance; IR, insulin resistance; MIR, muscle insulin resistance; LIR, liver insulin resistance; IR, OGTT, oral glucose tolerance test; OP, optimal; SUB, suboptimal; LR, low risk; LI, lifestyle intervention; HR, high risk; ISI, insulin sensitivity index; INT, intensified; CONV, conventional; TE, total energy; 2hPPG, 2 h postprandial glucose.
One of the first frameworks published to deliver personalised nutrition using metabotypes targeted generally healthy individuals in Ireland(Reference O'Donovan, Walsh and Nugent50). By applying k-means clustering to four routinely measured markers of metabolic health (TAG, total cholesterol, HDL-C and glucose), three metabotypes were obtained. To deliver nutrition advice, decision tree algorithms were designed with the incorporation of metabotype information and individual BMI, waist circumference and blood pressure. The metabotypes were later successfully replicated in the German cohort KORA with additional analyses revealing differences in the habitual dietary intake across metabotypes and, most importantly, in the incidence of cardiometabolic diseases(Reference Riedl, Hillesheim and Wawro20). Metabotype 3, initially characterised by the most unfavourable metabolic profile, was further characterised by the poorest diet and the highest incidence of cardiometabolic diseases. These findings supported further development of the framework and an update of the dietary messages was performed to incorporate more specific recommendations on nutrient intake and management of cardiometabolic diseases(Reference Hillesheim, Ryan and Gibney51). Compared to individualised advice, the updated metabotype framework showed good performance with an agreement of 83 % between dietary messages. Application of this framework to deliver personalised nutrition is currently under study using a 12-week RCT (n 107)(Reference Brennan52). The primary outcome will determine if personalised nutrition advice delivered through the metabotype framework is more effective compared to population-level nutrition advice at improving dietary quality.
The PERSonalized Glucose Optimization Through Nutritional Intervention (PERSON) study is currently underway to examine the ability of metabotypes to deliver nutrition advice(Reference Gijbels, Trouwborst and Jardon53). The 12-week RCT includes 240 overweight or obese individuals to investigate the effects of a macronutrient intervention on glucose metabolism parameters according to metabotypes of tissue-specific insulin resistance. Using cut-off values of muscle insulin sensitivity index and hepatic insulin resistance index two metabotypes are defined: muscle insulin resistance and liver insulin resistance. In each metabotype, participants are randomised to a diet considered optimal or suboptimal for their metabolic profile: an optimal diet for muscle insulin resistance and suboptimal for liver insulin resistance is high in monounsaturated fat and a suboptimal diet for muscle insulin resistance and optimal liver insulin resistance is low in total fat and high in protein and fibre. The primary outcome is the difference in change in disposition index, a measure of β-cell function, between participants who will receive their hypothesised optimal or suboptimal diet. The results from the study will be important to support the use of metabotype approaches for the development of tailored diets to improve glucose homoeostasis.
With a focus on improving body composition of overweight and obese individuals through personalised nutrition based on metabotypes, the efficacy of the PREVENTOMICS platform was compared to generic dietary advice(Reference Aldubayan, Pigsborg and Gormsen54). Using fifty-one urine and blood biomarkers and thirty-five single-nucleotide polymorphisms assessed in saliva samples, the PREVENTOMICS algorithm calculates scores for each individual in five metabolic processes that name the metabotypes: oxidative stress, inflammation, carbohydrate metabolism, lipid metabolism and gut microbiota metabolism. Individuals are classified into the metabotype with their highest score and dietary plans are created with the metabotype characteristics. Details of the algorithm were not disclosed due to an intellectual property rights application. In a 10-week RCT, the platform was tested with 100 individuals and the primary outcome was the difference in the change in fat mass between personalised and generic groups. To implement the dietary plans, all participants received two isoenergetic vegetarian meals daily and were referred to an app with recipes for further meals. Following the intervention, both personalised and generic groups presented reductions in fat mass, body weight, diastolic blood pressure, total cholesterol, oxidised LDL-C, insulin, homoeostatic model assessment for insulin resistance, leptin and creatinine. However, there were no differences between groups and no metabotype-specific effects indicating that the metabotype-based diet personalisation performed by the PREVENTOMICS platform did not further improve body composition, weight homoeostasis and cardiometabolic risk factors compared to a plant-based and generally healthy diet.
Finally, the Prediabetes Lifestyle Intervention Study (PLIS) investigated the impact of different intensities of an LI (dietary and exercise advice) on metabotypes of low risk and high risk for diabetes(Reference Fritsche, Wagner and Heni55). The 12-month RCT included 1105 individuals with prediabetes that were classified into the metabotypes using cut-off values of insulin secretion, insulin sensitivity and liver fat content. High-risk individuals were randomised to an intensified or conventional LI and low-risk individuals were randomised to a conventional LI or control. The conventional LI consisted of eight coaching sessions with nutrition and exercise advice and the intensified LI consisted of double the amount. The primary outcome was the difference in postprandial glucose between intervention groups within each metabotype. In the high-risk metabotype, the intensified LI resulted in larger reductions in postprandial glucose, liver fat content, cardiometabolic risk score and BMI and higher insulin sensitivity compared to the conventional LI. In the low-risk metabotype, there was no difference between groups in postprandial glucose, but a lower BMI and fasting glucose in the conventional LI compared to the control group. This study demonstrates different responses of prediabetes risk-based metabotypes to LI and suggests that targeted lifestyle approaches may be beneficial for the prevention of diabetes.
In conclusion, recent years have seen a heightened interest in the use of metabotyping to tailor nutrition advice but evidence of the effectiveness of such an approach to improving health outcomes is still insufficient. Building this evidence base will be important prior to the application of metabotyping into clinical practice.
Conclusions
Metabotyping can classify individuals into subgroups with meaningful metabolic profiles. Recent applications in nutrition, which are corroborated by other fields, show clearly that in longitudinal studies such metabotypes have different health outcomes. The challenge now is to harness this information to tailor preventative nutrition advice to the metabolic phenotype. However, there is a paucity of follow-up intervention studies with this focus. Such interventions are paramount to further development and acceptance of the approach. Furthermore, work is needed to distil the panels of markers used into routinely measured markers that will facilitate the potential uptake and keep costs affordable. Ensuring that the frameworks developed are low cost with scientific evidence supporting their use will be key to implementation. Engagement with healthcare professionals will be essential to facilitating further use and development of the metabotype concept for the delivery of tailored nutrition advice.
While the application of metabotypes to tailor nutrition advice is very promising, the evidence in terms of RCTs is lacking and needs to be urgently addressed. Carefully designed intervention studies are needed to demonstrate efficacy in terms of improving health outcomes.
Acknowledgements
The authors acknowledge the Irish section of the Nutrition Society for inviting the present review paper as part of the postgraduate review competition.
Financial Support
This work was supported by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (E. H., grant number 88881.174061/2018-01) and the European Research Council (L. B., grant number 647783).
Conflict of Interest
None.
Authorship
E. H. and L. B. contributed to the conception and design of the manuscript, E. H. drafted the manuscript and L. B. revised the manuscript.