Pregnancy represents a period of rapid tissue growth of maternal and fetal tissues that is associated with increased energy and nutrient requirements. Maternal nutrition during pregnancy, as part of the ‘first 1000 days’, is widely recognised as being essential for optimal offspring development, reducing lifelong disease burden and for general health throughout life(Reference McDonald, Thorne-Lyman, Karakochuk, Whitfield, Green and Kraemer1). In particular, folate plays a critical role in pregnancy as it is required for C1 metabolism, a network of metabolic pathways involved in nucleotide synthesis, DNA repair, methylation reactions and neurotransmitter synthesis and thus is essential during periods of rapid tissue growth(Reference Bailey, Stover and McNulty2). In early pregnancy, there is conclusive evidence that periconceptional folic acid (FA) supplementation has a beneficial effect in preventing neural tube defects (NTD)(3, Reference Czeizel and Dudas4). It is almost 30 years since two large clinical trials proved that periconceptional FA supplementation of mothers was essential in the prevention of NTD. This led many countries worldwide to introduce mandatory FA food fortification programmes(5), whereas other countries (most notably within Europe) have chosen public health strategies promoting periconceptional FA supplementation only. Apart from NTD, emerging evidence suggests that maternal folate throughout pregnancy may have other roles in offspring health, including neurodevelopment and cognitive performance in the first decade of life. This review will explore the evidence linking maternal folate status with offspring health and will consider the associated biological mechanisms. In addition, the challenge of translating the evidence to public health, and somewhat controversial policies, will be considered.
Role of folate in human health
Metabolic role of folate
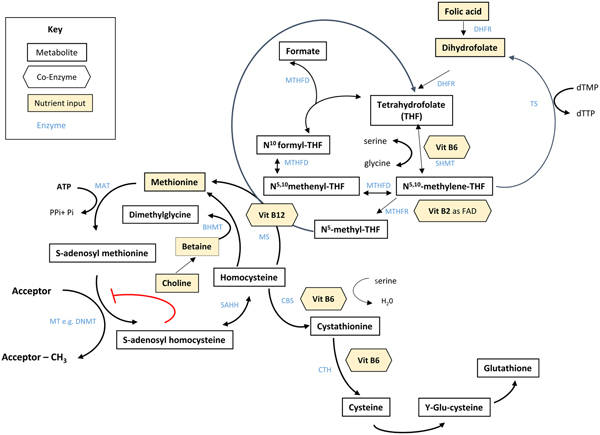
Folate plays an essential role in C1 metabolism where it acts as a cofactor in DNA synthesis and repair, methylation and amino acid reactions (Fig. 1). Within this network, folate coenzymes function in mediating the transfer and utilisation of C1 units in metabolic pathways involving interaction with vitamin B12, vitamin B6 and riboflavin(Reference Bailey, Stover and McNulty2). Reduced folates enter the C1 cycle as tetrahydrofolate (THF) which acquires a carbon unit from serine in a vitamin B6-dependent reaction and subsequently forms 5,10-methylene THF, which is required for the synthesis of nucleic acids, or converted to 5-methyl THF. Methylenetetrahydrofolate reductase is the riboflavin (FAD)-dependent enzyme that catalyses the reduction of 5,10-methylene THF to 5-methyl THF. Within the methionine cycle, 5-methyl THF is required for the remethylation of homocysteine to methionine via the vitamin B12-dependent enzyme methionine synthase. Methionine, in turn, is required for the generation of S-adenosylmethionine (SAM), the essential methyl donor for innumerable genomic and nongenomic methylation reactions required for the nervous system(Reference Bailey, Stover and McNulty2). This pathway is essential for the methylation of DNA, by DNA methyltransferases using SAM as a cofactor, which can play a key role in controlling gene expression in a process referred to under the umbrella term epigenetics(Reference Armstrong6). Methylation is also essential in the synthetic pathways of neurotransmitters (dopamine, noradrenaline and serotonin), myelination and phospholipids and is thus important for normal brain function. Over the past 40 years, the association between neurology and B-vitamin status has been extensively investigated, with evidence that folate deficiency can lead to aberrant methylation and could, in turn, affect neurocognition(Reference Reynolds7).
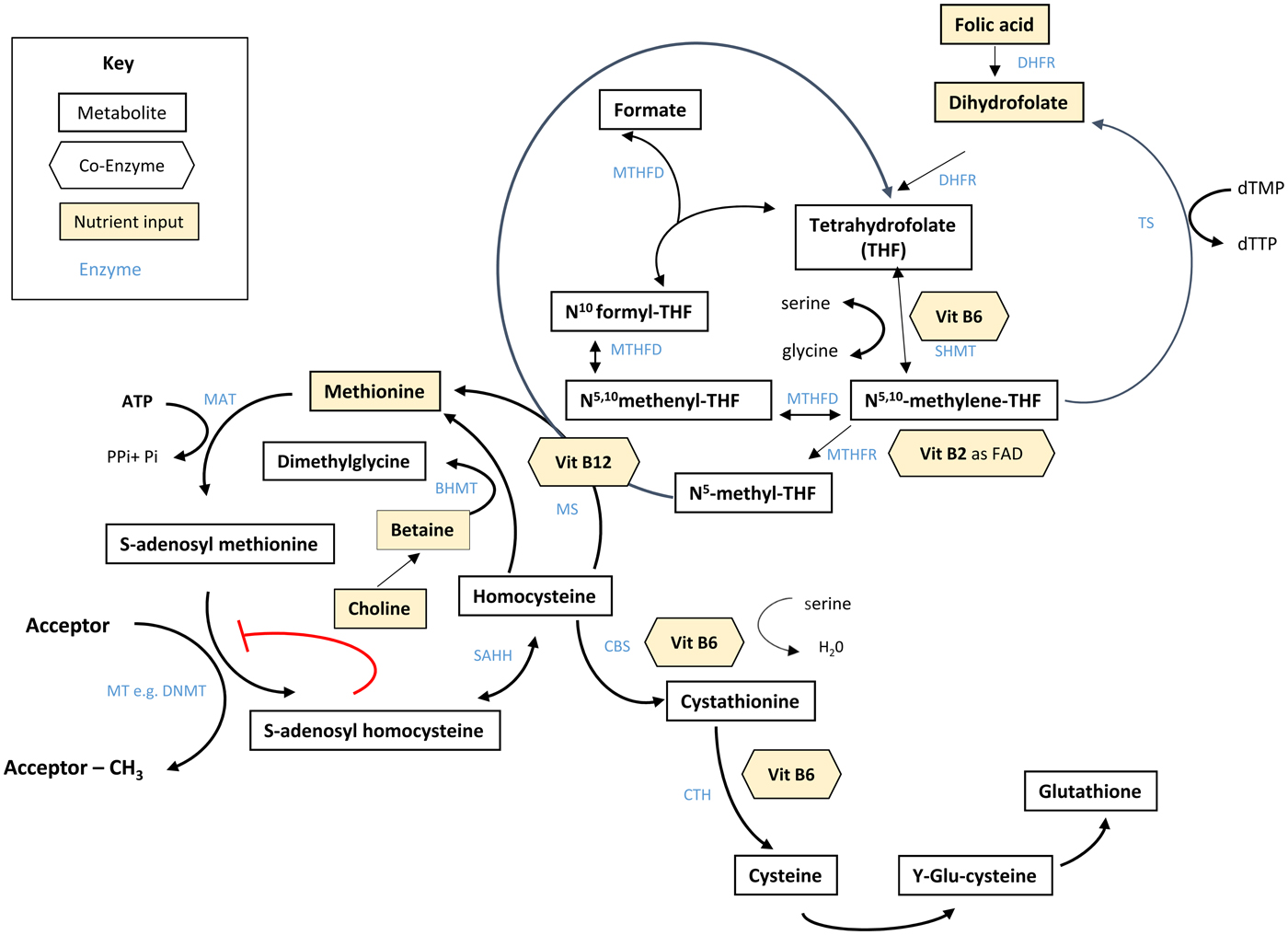
Fig. 1. (Colour online) Overview of C1 metabolism. BHMT, betaine homocysteine methyltransferase; CBS, cystathionine-β-synthase; CTH, cystathionine gamma-lyase; DHFR, dihydrofolate reductase; dTMP, deoxythymidine monophosphate; dTTP, deoxythymidine triphosphate; FAD, flavin adenine dinucleotide; DNMT, RNA methyltransferase; MAT, methionine adenosyltransferase; MS, methionine synthase; MT, methyl transferases; MTHFR, methylenetetrahydrofolate reductase; MTHFD, methylenetetrahydrofolate dehydrogenase; SAHH, S-adenosyl homocysteine hydrolase; SHMT, serine hydroxymethyltransferase; TS, thymidylate synthase. (Adapted from James et al.(Reference James, Sajjadi and Tomar134) Epigenetics, nutrition and infant health. In The Biology of the First 1000 Days, (KD Karakochuk, KC Whitfield, TJ Green, K Kraemer, editors). Florida: CRC Press).
Role of folate in pregnancy
The effect of folate status on pregnancy outcomes has long been recognised since the original discovery of folate by Lucy Wills in 1931 when marmite or other yeast extracts were found to be effective for the treatment of macrocytic anaemia in pregnant women(Reference Wills8); later it was discovered that the active factor was folate.
Pregnancy is recognised as a time when folate requirements are increased to sustain the demand for rapid cell division and growth of fetal, placental and maternal tissue. This reflects the critical role folate plays in DNA, RNA and protein synthesis(Reference Bailey, Stover and McNulty2). Along with the physiological changes related to the growth of maternal and fetal tissues, there is an expansion of plasma volume by 50 % compared with an increase in the erythrocyte mass by 25 %(Reference Milman, Byg and Agger9), increasing the demand for folate. Globally, the most common causes of anaemia of pregnancy (defined as a Hb concentration of less than 11 g/dl, at any point during pregnancy(10)) are iron and/or folate deficiency, arising from increased fetal requirements and frequently aggravated by decreased maternal nutrient reserves(Reference Lee and Okam11). Numerous studies have illustrated that the prevalence of folate deficiency increases with advancing gestational age(Reference Ackurt, Wetherilt and Loker12). A more recent trial in later pregnancy, however, showed that the decline in serum and erythrocyte folate concentrations and increase in plasma homocysteine concentrations, that otherwise occur as pregnancy progresses, can be prevented by continued FA supplementation (0·4 mg/d) in the second and third trimesters of pregnancy(Reference McNulty, McNulty and Marshall13); which in turn may prevent anaemia in later pregnancy(Reference Lassi, Salam and Haider14).
Observational studies have suggested that low maternal folate status is also associated with an increased risk of other adverse pregnancy outcomes including pre-eclampsia, gestational hypertension and preterm delivery(Reference Lindblad, Zaman and Malik15–Reference Czeizel, Puhó and Langmar17), while improved folate may help to prevent these conditions. In a study of 3000 Canadian women, supplementation of multivitamins containing FA was associated with a reduced risk of pre-eclampsia(Reference Wen, Chen and Rodger18), while in a study of 215 Korean pregnant women, FA supplementation was associated with significantly lower risk of pre-eclampsia(Reference Kim, Ahn and Ryu19). In contrast, one very recent international randomised trial found that high dose of FA in pregnancy did not reduce pre-eclampsia in high-risk pregnancy(Reference Wen, White and Rybak20). Notably, this study did not account for the common MTHFR C677 T polymorphism which is associated with a significantly increased risk of hypertension and hypertension in pregnancy(Reference McNulty, Strain and Hughes21).
The demand for folate is also increased during lactation to support neonatal growth and development(Reference Tamura and Picciano16). Folates are actively transported across the mammary epithelium, thereby allowing breast-milk folate concentration to be maintained and preventing folate insufficiency in breast-fed infants, but this is at the expense of maternal folate status in the absence of supplementation(Reference Mackey and Picciano22). Only in the case of frank maternal folate deficiency is milk folate reported to decline to critically low concentrations(Reference Metz, Zalusky and Herbert23).
Maternal folate and offspring health
Neural tube defects and other congenital abnormalities
Since the early 1990s, conclusive evidence has existed that periconceptional FA supplementation prevents the first occurrence(Reference Czeizel and Dudas4) and recurrence of NTD(3). This has led to worldwide recommendations that have been in place since 1992(24), for women of childbearing age to take 0·4 mg/d FA from before pregnancy until the end of the first trimester. NTD, including spina bifida, anencephaly, encephalocele and hydrocephalus, are major birth defects that can lead to miscarriage, stillbirth, or to lifelong and usually severe disabilities. NTD are the largest group of anomalies of the central nervous system characterised by incomplete closure of the embryonic neural tube and are among the most significant congenital causes of morbidity and mortality in infants worldwide(Reference Zaganjor, Sekkarie and Tsang25, Reference Botto, Moore and Khoury26). The conclusive evidence of the protective effect of FA comes from two randomised controlled trial (RCT), the first of which demonstrated that periconceptional supplementation at 4 mg/d FA in women with a history of NTD (n 1195), decreased the recurrence by 70 %(3). The second trial by Czeizel and Dudas(Reference Czeizel and Dudas4) in over 4000 women, showed that periconceptional multiple micronutrient supplementation containing 0·8 mg/d FA prevented the first occurrence of NTD.
These intervention studies, although not designed to test birth defects other than NTD, yielded additional information on other congenital abnormalities. The aforementioned Czeizel and Dudas trial(Reference Czeizel and Dudas4) was the subject of a subsequent analysis which found that the total rate of all major congenital abnormalities (including heart defects, oral facial clefts and urinary tract anomalies) were significantly reduced in women using FA-containing multivitamins during the periconceptional period(Reference Czeizel27). Consistent with these findings, FA fortification in Canada has been associated with an 11 % reduction in the prevalence of overall congenital heart defects(Reference Liu, Joseph and Luo28). A recent meta-analysis of fifteen studies from countries worldwide reported a decreased risk of cleft lip, with or without cleft palate, when orofacial cleft prevalence was examined in pre- v. post-FA fortification periods(Reference Millacura, Pardo and Cifuentes29).
Offspring brain development
Recent advances in behavioural neuroscience have shown the important roles that nutrition plays in brain development(Reference Fernstrom30–Reference Nyaradi, Li and Hickling33). Brain development begins at the very early stages of fetal life and continues after birth through early life. Initially, brain cells are formed followed by cell migration and differentiation, and the development of synapses to enable cells to communicate with one another(Reference Irwin, Pentieva and Cassidy34). Myelin is the supportive tissue that surrounds and protects the nerve axons and facilitates communication. Nutrient deficiencies, such as inadequate folate intake, can interfere with early brain development and function, resulting in neuroanatomical, neurochemical, or neurometabolic changes that are expressed by restricting the myelination and synaptic connectivity(Reference Black35) as well as changes in tissue levels of neurotransmitters (e.g. serotonin, dopamine, norepinephrine and acetylcholine). The functional consequences of these alterations vary, depending on the specific nutritional deficiency and the timing of the deficiency relative to the development of the neurological structures(Reference Roffman36). The last trimester of pregnancy until 2 years after birth, is a critical period of rapid growth and development of certain regions of the brain such as cortical and subcortical grey matter(Reference Isaacs32, Reference Hasegawa, Houdou and Mito37, Reference Gilmore, Shi and Woolson38).
Myelination of the brain, which is most intensive from mid-gestation through the second year of life but continues through puberty, may be specifically vulnerable to B-vitamin deficiency(Reference Black35). In infants, B-vitamin deficiencies have been associated with demyelination and brain atrophy(Reference Lövblad, Ramelli and Remonda39). Thus the continuation of FA supplementation after the first trimester (i.e. after the recommended period for the prevention of NTD) may also be an important period for optimal folate status and prenatal brain development(Reference Irwin, Pentieva and Cassidy34, Reference Roffman36, Reference McGarel, Pentieva and Strain40). Thus maternal folate nutritional status can influence both structural and functional development of the brain(Reference Georgieff, Brunette and Tran41), while folate insufficiencies in pregnancy may result in lasting changes in brain function.
Maternal folate and offspring cognitive performance
The effect of maternal folate during pregnancy on cognitive performance of the offspring has been investigated in several studies, with evidence to date coming predominantly from observational research (Table 1)(Reference Julvez, Fortuny and Mendez42–Reference Valera-Gran, Navarrete-Muñoz and De La Hera57). Most of these studies have focused on reported FA supplement use or folate status of mothers in early pregnancy, whereas later pregnancy (i.e. beyond first trimester) has rarely been investigated. A number of studies in early pregnancy have shown positive associations between self-reported FA supplement use and cognitive performance in the child(Reference Julvez, Fortuny and Mendez42, Reference Roth, Magnus and Schjølberg45, Reference Villamor, Rifas-Shiman and Gillman46). These findings are in general agreement with studies that found reduced cognitive ability in the offspring of mothers with suboptimal folate status(Reference Schlotz, Jones and Phillips44, Reference Murphy, Fernandez-Ballart and Molloy49). Likewise, one recent systematic review of fourteen studies of maternal nutritional status in pregnancy and offspring cognitive function concluded that low maternal folate status was associated with poorer offspring cognitive function(Reference Veena, Gale and Krishnaveni58).
Table 1. Summary of observational studies investigating the association between maternal folate status and cognitive performance of the offspring

MSCA, McCarthy Scales of Children's Abilities; FA, folic acid; BSID, Bayley Scales of Infant and Toddler Development; SDQ, Strengths and Difficulties Questionnaire; LGS, language grammar scale; PPVT, Peabody Picture Vocabulary Test; WRAVMA, Wide Range Assessment of Visual Motor Abilities; WRAML, Wide Range Assessment of Memory and Learning; KBIT, Kaufman Brief Intelligence Test; WPPSI, Weschler Preschool and Primary Scale of Intelligence; tHcy, total plasma homocysteine; DDST, Denver Developmental Screening Test; DAS, Differential Ability Scales; K-ABCKM, Kaufman Assessment Battery for Children; GW, gestational week, NEPSY, A Developmental NEuroPSYchological Assessment.
Compared with the aforementioned studies in early pregnancy, evidence provided by Gross et al.(Reference Gross, Newberne and Reid50) over 40 years ago showed that children born to mothers with diagnosed megaloblastic anaemia in the third trimester of pregnancy had abnormal neurodevelopment and lower intellectual abilities compared with infants born to mothers with optimal folate status. Several decades later, a longitudinal study of 256 mother–child pairs linked maternal folate deficiency in later pregnancy with reduced brain volume in the children aged 6–8 years, as measured using MRI(Reference Ars, Nijs and Marroun56). Further to this, a study investigating the impact of maternal blood folate, vitamin B12 and homocysteine concentrations at the 30th gestational week, showed that higher maternal folate status during later pregnancy predicted better cognitive performance in children aged 9–10 years(Reference Veena, Krishnaveni and Srinivasan52). There have however been two longitudinal observational studies that found no significant associations between blood folate status in later pregnancy and cognitive performance(Reference Tamura, Goldenberg and Chapman51) or infant neurodevelopment(Reference Wu, Dyer and King54).
A number of studies in this area have investigated offspring cognition in relation to the reported use of FA above the recommended dose of 0·4 mg/d. One such study by Chatzi et al.(Reference Chatzi, Papadopoulou and Koutra53) found that self-reported FA supplement usage of 5 mg/d in later pregnancy was associated with enhanced vocabulary and verbal skills of the offspring in the first 2 years of life. In contrast, another European study found that FA supplement usage of >1 mg/d as reported by mothers was associated with reduced verbal and cognitive development, compared with the children of mothers with FA intakes of 0·4 mg/d during the second and third trimesters of pregnancy(Reference Valera-Gran, García De La Hera and Navarrete-Muñoz55, Reference Valera-Gran, Navarrete-Muñoz and De La Hera57). In an effort to validate self-reported FA supplement use by mothers, Chatzi et al.(Reference Chatzi, Papadopoulou and Koutra53) collected cord blood samples and showed that mothers who reported high dose FA supplement usage gave birth to neonates with higher erythrocyte folate concentrations in cord blood.
A major limitation in the aforementioned studies is that they are observational and thus, by design, cannot confirm whether a causal relationship between maternal folate nutrition and offspring cognitive performance exists. For example, some studies have found supplement usage to be more frequent among pregnant women at lowest nutritional risk(Reference Malek, Umberger and Makrides59), raising the possibility that FA usage may simply be a marker of positive health considerations and that another nutrient (or non-nutritional factor) could explain the observed relationship. Supplement usage is reported to be higher in pregnant women who are older, have higher household incomes, with higher educational attainment, have planned their pregnancy, have breastfed their child, live with a partner, do not smoke and have a healthier weight(Reference Watson, Brown and Davey60–Reference Malek, Umberger and Makrides65); any one of these factors could actually explain the observed relationship between maternal FA supplement usage and offspring cognition.
A randomised trial could provide evidence of a causative link between maternal folate and offspring cognition, but most available RCT have investigated the effect of multiple micronutrient supplements containing FA(Reference Dobó and Czeizel66–Reference Prado, Sebayang and Apriatni71), as shown in Table 2. The only RCT to date to look at the specific effect of 0·4 mg/d FA in isolation was conducted at this centre, namely the FASSTT trial (ISRCTN19917787)(Reference McNulty, McNulty and Marshall13) and provided a unique opportunity to specifically investigate the effect of FA supplementation in the second and third trimesters on subsequent cognitive performance of the child. The results showed that children of mothers supplemented with FA throughout pregnancy performed better in the cognitive domain at age 3 years(Reference Pentieva, McGarel and McNulty72) and the verbal domain at 7 years(Reference McGarel, McNulty and Strain73).
Table 2. Summary of randomised trials investigating the effect of maternal folic acid supplementation and cognitive performance of the offspring

FA, folic acid; GW, gestational week; UNIT, Universal Nonverbal Intelligence Test; MABC, Movement Assessment Battery for Children; 5-MTHF, 5-methyltetrahydrofolate; K-ABCKM, Kaufman Assessment Battery for Children; BSID, Bayley Scales of Infant and Toddler Development; MMN, multiple micronutrient; WPPSI, Weschler Preschool and Primary Scale of Intelligence; EEG, electroencephalography.
FA dosage is 0·4 mg/d, unless otherwise stated.
These findings are in agreement with the results of animal studies, whereby continuation of maternal FA supplementation increased serum folate concentrations in pregnant rat dams and improved neurodevelopment in their pups, from newborns to adulthood(Reference Wang, Li and Li74). A histological investigation from the same study found that continued FA supplementation throughout pregnancy stimulated hippocampal neurogenesis in the offspring by increasing proliferation and neuronal differentiation of neural stem cells and also by enhancing synaptogenesis in the cerebral cortex(Reference Wang, Li and Li75). Furthermore, offspring of pregnant mice fed an FA-deficient diet during gestation had a reduction of progenitor cells in the fetal neocortex(Reference Craciunescu, Brown and Mar76) which is the part of the brain responsible for complex behaviours such as cognition, attention and social competence.
Overall, the evidence from human and animal studies appears to support the role of maternal folate status in influencing the cognitive performance of the child. In addition to cognitive health, there are also human studies linking maternal folate throughout pregnancy with psychosocial behaviour(Reference Henry, Cassidy and Mclaughlin77) and cerebral cortex thickness in youths(Reference Eryilmaz, Dowling and Huntington78), which warrant further investigation. In summary, the totality of evidence in this area is promising, but remains inconclusive, given that the vast majority of human studies are observational and thus inherently limited.
Epigenetic mechanisms linking maternal folate with offspring health
Although the precise biological mechanism explaining the effect of FA during pregnancy on neurodevelopment of the child is unknown, it must involve the essential role of folate in C1 metabolism encompassing a complex network of interdependent pathways that support a variety of processes, including myelination, neurotransmitter synthesis and epigenetics, which in turn may impact neurodevelopment(Reference Black35, Reference Gabbianelli and Damiani79) (Fig. 1). Epigenetic marks, in particular, and specifically DNA methylation, have been proposed as plausible mechanisms underlying associations between folate and various disease outcomes such as NTD, cardiometabolic disorders and early life development(Reference Kok, Steegenga and Mckay80).
Epigenetics refers to histone modification, RNA interference or DNA methylation which can exert heritable changes in gene expression that occur without altering the underlying DNA sequence(Reference Armstrong6). DNA methylation is the most widely studied and understood epigenetic mechanism for gene regulation and is dependent upon the supply of methyl donors provided by folate and the metabolically related B-vitamins via the production of SAM within C1 metabolism (Fig. 1). SAM is an essential methyl donor for DNA and is therefore important for transcriptional regulation, thus folate deficiency could lead to aberrant gene expression and various consequential health outcomes(Reference James, Sajjadi and Tomar81). Early life development, ranging from preconception to childhood, is considered a critical window characterised by rapid DNA methylation changes, pronounced susceptibility to environmental factors and programming of epigenetic marks that may have long-lasting health effects(Reference Numata, Ye and Hyde82).
There has been growing interest in the importance of maternal folate status, DNA methylation and offspring neurodevelopment(Reference Irwin, Pentieva and Cassidy34, Reference Gabbianelli and Damiani79). One cohort study of women who reported using FA supplements after the 12th gestational week of pregnancy (n 913) found that FA was associated with lower methylation in cord blood for both LINE-1 and PEG3, and higher methylation for IGF2 (Reference Haggarty, Hoad and Campbell83). The largest study to date (n 1988), investigated epigenome-wide DNA methylation in newborns from two European pregnancy cohorts and reported an inverse association between maternal plasma folate during pregnancy and differential DNA methylation in cord blood across genes involved in folate biology and neurodevelopmental processes(Reference Joubert, den Dekker and Felix84). Another study showed that maternal periconceptional FA use (as reported by mothers) was associated with increased methylation of IGF2 in the offspring when measured at age 17 months(Reference Steegers-Theunissen, Obermann-Borst and Kremer85). However, a comprehensive review concluded that associations between maternal folate exposure and the offspring methylome were generally inconsistent, likely as a result of methodological differences between published studies, including differences in the form of folate used, the timing of exposure, baseline folate status, underlying genotype and the genomic region affected(Reference James, Sajjadi and Tomar81).
The main limitation of studies in this area is that they are observational and therefore cannot provide evidence of a causal relationship between maternal folate during pregnancy and DNA methylation effects in offspring. The only randomised trial to date was carried out at this centre and showed significant folate-related changes in DNA methylation of the retrotransposon LINE-1 and candidate genes related to brain development such as IGF2 and BDNF in the newborn of mothers who received 0·4 mg/d FA compared with placebo in the second and third trimesters(Reference Caffrey, Irwin and McNulty86). These findings have also been supported by the results of animal experiments. One such animal study investigated DNA methylation in the brain and found that FA supplementation throughout pregnancy significantly increased brain folate concentrations in the newborn pups, while brain global DNA methylation incrementally decreased and was the lowest in pups whose mothers were supplemented with FA throughout their entire pregnancy(Reference Ly, Ishiguro and Kim87). The findings offer a potential biological mechanism linking maternal folate status with neurodevelopment of the offspring, but this requires investigation using a genome-wide approach to more fully explore the underlying mechanisms.
In addition to maternal folate, vitamin B12 status has also been found to be a significant predictor of gene-specific DNA methylation in the offspring. Using a two-step ‘Mendelian randomisation study’ approach with data from the Avon Longitudinal Study of Parents and Children cohort, an effect of maternal serum vitamin B12 concentrations on cord blood DNA methylation, and an effect of vitamin B12-responsive DNA methylation changes on children's cognition at 8 years, were identified(Reference Caramaschi, Sharp and Nohr88). The finding that vitamin B12 may also influence DNA methylation in a similar way to folate is not surprising as it acts synergistically with folate within the C1 metabolic cycle and both vitamins are required for the generation of SAM(Reference Bailey, Stover and McNulty2) (Fig. 1).
Although the link between maternal nutrition and offspring health has been extensively studied, understanding of how the paternal diet could influence offspring health remains relatively under-investigated. One recent review, however, concluded that suboptimal paternal nutrition around the time of conception can play an important role in offspring health(Reference Fleming, Watkins and Velazquez89). In particular, the role of paternal nutrition in relation to sperm quality is emerging as potentially mediating offspring health(Reference Palmer, Bakos and Owens90, Reference Sinclair and Watkins91). Mechanistically, both direct (sperm quality, epigenetic status, DNA integrity) and indirect (seminal fluid composition) paternal characteristics have been identified; in mice, these mechanisms have been shown to affect offspring development across multiple generations(Reference Cropley, Eaton and Aiken92). In terms of the epigenetic effects, both DNA methylation(Reference Radford, Ito and Shi93) and small RNA species(Reference Cropley, Eaton and Aiken92) have been implicated as agents for the transmission of effects via the sperm.
So far, compelling evidence has suggested a role for epigenetics and DNA methylation in explaining the effects of nutrition in pregnancy on long-term offspring health outcomes. Folate-mediated epigenetic changes in genes related to brain development and function offer a biological basis to link maternal folate with offspring cognitive effects. Although this area of research is still in its infancy, future studies from RCT cohorts using an epigenome-wide approach will be necessary to more fully explore the underlying mechanisms.
Optimising folate status in women of reproductive age
Food folates, folic acid and bioavailability
There are three options to achieve optimal folate status in individuals and in populations, namely through naturally-occurring food folates, fortified foods and supplements. Food folates exist predominantly in the polyglutamyl form and are converted to monoglutamates for absorption, whereas FA, the synthetic vitamin form found in fortified foods and supplements, is a monoglutamate. In addition, natural folates are reduced molecules, whereas FA is fully oxidised(Reference Bailey, Stover and McNulty2). As a result, naturally occurring food folates show incomplete bioavailability compared with FA at equivalent levels of intake(Reference McNulty and Pentieva94). Apart from their limited bioavailability once in the body, food folates are inherently unstable during cooking, and this can substantially reduce the folate content of this food before they are even ingested(Reference McKillop, Pentieva and Daly95). In contrast, FA provides a highly stable and bioavailable form of the vitamin. The bioavailability of FA is assumed to be 100 % when ingested as a supplement, while FA in fortified food is estimated to have about 85 % the bioavailability of FA supplements(Reference Pfeiffer, Rogers and Bailey96). Folate intakes and recommendations in the USA and other countries are therefore now expressed as Dietary Folate Equivalents, a calculation that considers the greater bioavailability of FA compared with naturally occurring food folates(97).
Owing to the instability and poor bioavailability of natural food folates, the potential to optimise folate status through food folates alone is relatively ineffective, whereas intervention with FA supplements or FA fortified food has been shown to be highly effective in optimising folate biomarker status in women of reproductive age(Reference Cuskelly, McNulty and Scott98).
Folic acid recommendations for neural tube defect prevention
Once conclusive evidence had become available to show that FA supplementation could prevent the first occurrence(Reference Czeizel and Dudas4) and recurrence of NTD(3), committees worldwide produced recommendations for women of childbearing age to take 0·4 mg/d FA from before pregnancy until the end of the first trimester(24, 99). These recommendations, which have been in place for almost 30 years, are challenging for a number of reasons. An estimated 50 % of pregnancies are unplanned(Reference Mills and Dimopoulos100) and for many women, the very early stage of pregnancy (when the neural tube is closing) may have passed before supplementation is even started. Therefore, in many cases, the malformations of NTD may have occurred before a woman even knows that she is pregnant.
There is evidence from nearly 500 000 pregnant women that only 31 % took FA supplements before pregnancy, with women under 20 years of age and non-caucasian women being the least likely to take FA as recommended(Reference Bestwick, Huttly and Morris101). Therefore, the public health measure of recommending FA supplements before pregnancy has substantial limitations and is putting young women and those in ethnic minorities at a particular disadvantage(Reference Bestwick, Huttly and Morris101). Research from Northern Ireland(Reference McNulty, Pentieva and Marshall102) and the Republic of Ireland(103) indicates low levels of compliance among Irish women surveyed during pregnancy, between 14 and 44 % reporting to have taken FA supplements as recommended in the periconceptional period. This is of concern given that the benefit of FA supplementation in preventing NTD is confined to those women (the minority) who follow the recommendations correctly.
The measurement of erythrocyte folate in women of reproductive age is a useful way to assess NTD risk within populations on the basis of the known continuous dose–response inverse relationship between maternal erythrocyte folate concentrations and NTD(Reference Daly, Kirke and Molloy104). On this basis, the WHO has established guidelines for optimal erythrocyte folate concentrations of 906 nmol/l in women of reproductive age for the prevention of NTD(105). In the UK, where there is voluntary (but not mandatory) fortification of foods with FA, the percentage of women with insufficient erythrocyte folate concentrations (<906 nmol/l) to prevent folate-responsive NTD is estimated to be 83 % in Northern Ireland, 81 % in Scotland and 79 % in Wales(106). Also, evidence from the National Adult Nutrition Survey in Ireland showed that non-consumers of FA from fortified food or supplements were at particularly high risk of suboptimal folate status, again using the cut-point of 906 nmol/l erythrocyte folate to define optimal status(Reference Hopkins, Gibney and Nugent107).
Folic acid fortification and neural tube defect prevalence
The indisputable protective role of FA in the prevention of NTD, coupled with the low compliance of women to FA recommendations, has stimulated the option of mandatory FA fortification, a policy now in place in over 80 countries worldwide(5). Mandatory food fortification requires food manufacturers to add FA to certain foods (e.g. starch or grain products), whereas voluntary fortification allows FA to be added to foods at the discretion of manufacturers. A systematic review and meta-analysis of global birth prevalence of spina bifida by FA fortification status found that spina bifida prevalence was significantly lower in studies from countries where FA fortification was mandatory (3·4 per 10 000 live births) compared with countries where fortification was voluntary or non-existent (4·8 per 10 000 live births)(Reference Atta, Fiest and Frolkis108). Furthermore, evidence has shown that in 2017 only 18 % of FA-preventable NTD cases globally were prevented, resulting in 230 000 children with either spina bifida or anencephaly(Reference Kancherla, Wagh and Johnson109). In the USA, after the implementation of mandatory FA fortification, reported rates of NTD prevalence decreased by 35 %, from 10·7 to 7·0 NTD per 10 000 live births, preventing over 1300 NTD annually(Reference Parker, Mai and Canfield110–Reference Crider, Qi and Devine112). In Canada, the prevalence of NTD decreased by 46 %, from 15·8 per 10 000 births before FA food fortification to 8·6 per 10 000 births after implementation of mandatory fortification(Reference De Wals, Tairou and Van Allen113).
In contrast, public health strategies based on promoting increased awareness of the benefits of FA supplementation, as in place in European countries, have been shown to be largely ineffective(Reference Khoshnood, Loane and De Walle114–Reference Obeid, Pietrzik and Oakley116). Analysis of European data showed that average infant mortality with congenital anomaly was 1·1 per 1000 births, with higher rates where termination of pregnancy is illegal (Malta 3·0 and Ireland 2·1)(Reference Boyle, Addor and Arriola117). These rates have shown no downward trend over time, even with the introduction of government recommendations for FA usage, and NTD rates in 2011 were found to be comparable with that in 1991 (about 0·9 per 1000 births)(Reference Khoshnood, Loane and De Walle114, Reference Botto, Lisi and Robert-Gnansia115). As a result, one report investigating the period 2000–2010 estimated 1·6-fold higher NTD prevalence in European countries compared with countries with mandatory food fortification in place(Reference Obeid, Pietrzik and Oakley116).
Ireland is recognised as having one of the highest rates of NTD-affected pregnancies in the world and there are concerns that the incidence of NTD is increasing in recent years(Reference Botto, Lisi and Robert-Gnansia115). In 2016, following an extensive review, the Food Safety Authority of Ireland scientific committee published an updated report recommending that mandatory fortification of bread or starch with FA should be implemented(103). Similarly, The UK Scientific Advisory Committee on Nutrition has recently confirmed its longstanding advice that mandatory fortification of cereal flours with FA should be introduced for the prevention of NTD(118). Legislation to implement mandatory FA fortification has yet to be introduced in either country.
Current controversies and public health challenges
FA, the synthetic form of folate, is used for food fortification and supplementation purposes. Once ingested, FA is reduced by dihydrofolate reductase and after subsequent methylation, it is released in the systemic circulation as 5-methyl THF. However, the reduction of FA is a slow process that is influenced by individual variations in dihydrofolate reductase activity(Reference Bailey, Stover and McNulty2) and thus exposure to high oral doses of FA can result in the appearance of unmetabolised FA in the circulation(Reference Kelly, McPartlin and Goggins119). The latter is not a normal constituent of plasma or other tissues. On this basis, concerns have been raised regarding potential (although as yet unconfirmed) adverse health effects of unmetabolised FA arising in the circulation through high FA exposures from supplements and fortified foods.
One issue was the historical concern that long-term exposure to high dose FA intakes might mask the macrocytic anaemia of vitamin B12 deficiency, common in older people while allowing the associated irreversible neurologic symptoms to progress(Reference Dickinson120). Furthermore, analyses of National Health and Nutrition Examination Survey (1999–2002) data in the USA showed that in elderly participants with low vitamin B12 status, the presence of unmetabolised FA in serum was associated with worse cognitive performance compared with those with low vitamin B12 status and no detectable FA in the circulation(Reference Morris, Jacques and Rosenberg121). Subsequent studies have not been able to confirm such effects(Reference Clarke, Sherliker and Hin122, Reference Mills, Carter and Scott123), therefore these particular findings remain rather controversial. Other evidence has suggested that FA doses in excess of 1 mg/d may promote the growth of new or already existing but undiagnosed colorectal adenomas in those with pre-existing lesions(Reference Cole, Baron and Sandler124). One recent meta-analysis (involving 50 000 individuals) however concluded that FA supplementation neither increased nor decreased site-specific cancer within the first 5 years of treatment(Reference Vollset, Clarke and Lewington125), whilst one review reported decreases in cancer rates since the introduction of mandatory FA fortification in the USA(Reference Keum and Giovannucci126).
A number of observational studies conducted in countries with either mandatory or voluntary FA food fortification have found detectable amounts of unmetabolised FA in the circulation of pregnant women(Reference Sweeney, Staines and Daly127–Reference Plumptre, Masih and Ly130) and newborns(Reference Sweeney, Staines and Daly127, Reference Obeid, Kasoha and Kirsch129–Reference Sweeney, McPartlin and Weir132). Although it remains to be proven whether there are adverse effects associated with unmetabolised FA in the circulation, pregnancy may be of particular interest in this context as a vulnerable time of the life cycle. Moreover, the usage of FA supplements during pregnancy is widespread because FA is recommended worldwide from preconception until the end of the first trimester for protection against NTD, and in later pregnancy, it is often prescribed by obstetricians for the treatment and prevention of folate deficiency anaemia. The only randomised trial in this area, previously carried out by this research group, provides evidence that continuing FA supplementation at a dose of 0·4 mg/d throughout the second and third trimesters (over and above FA intakes through fortified foods), results in no detectable unmetabolised FA concentrations in cord blood, despite improving folate status of mothers and neonates(Reference Pentieva, Selhub and Paul133). Thus, in the event that adverse effects of unmetabolised FA are ever proven, this evidence indicates that the exposure of pregnant women to 0·4 mg FA/d will have little impact.
Even though the risk–benefit debate surrounding food fortification with FA continues among policymakers, the totality of the evidence at this time suggests that adverse effects associated with FA overexposure are unlikely at the generally low FA levels arising through mandatory food fortification.
Conclusions
Maternal FA supplementation before and in early pregnancy is known to have beneficial effects in the prevention of NTD. Emerging evidence suggests that it may also be beneficial for fetal brain development in later pregnancy. Mechanistically, the known role of folate in C1 metabolism and thus in methylation of proteins and DNA provides a biological basis to link maternal folate with offspring health mediated via epigenetic effects. However, this area of research is still in its infancy and the role of maternal folate status during pregnancy on the offspring and subsequent long-term health effects requires further investigation in carefully designed studies.
Although there are clear recommendations in place worldwide for the prevention of NTD through FA supplementation before and during early pregnancy, for many women the very early stage when the neural tube is closing may have passed before supplementation is even started. Thus, current health strategies in the UK, Ireland and the rest of Europe for the prevention of NTD (based on periconceptional FA supplementation only), have been shown to be largely ineffective. Mandatory food fortification with FA offers a solution that has proved to be highly effective in decreasing NTD cases in populations where it has been implemented, but this policy is controversial owing to concerns related to potential adverse effects of over-exposure to FA. In the absence of population-wide fortification and given the generally poor compliance of women with current FA recommendations, optimising folate status of mothers at the very early stage of pregnancy for protection against NTD remains challenging.
Financial Support
This work was supported by the HSC Research and Development Division of the Public Health Agency, Northern Ireland (Enabling Research Award STL/5043/14), joint funding (Grant Ref: ES/N000323/1 ‘EpiFASSTT’) from the Biology and Biological Sciences Research Council and the Economic and Social Research Council and from the Northern Ireland Department for Economy, which funded the PhD studentship for A. C. The funders had no role in the design, analysis or writing of this article.
Conflicts of Interest
None.
Authorship
A. C. drafted the manuscript; K. P., H. McN., C. P. W. and R. E. I. critically revised the manuscript for important intellectual content. All the authors have read and approved the final manuscript.