- CT
computerised tomography
- IF
intestinal failure
The term intestinal failure (IF) was introduced by Fleming and Remington(Reference Fleming, Remington and Hill1) and defined as a ‘reduction in functioning gut mass below the minimum necessary for adequate digestion and absorption of nutrients’. Initially, this definition was used interchangeably with the need for parenteral nutrition(Reference Shaffer2, Reference Lal, Teubner and Shaffer3). Since that time the definition has been broadened and is now recognised to occur when ‘gastrointestinal function is inadequate to maintain the nutrition and hydration of the individual without supplements given orally or intravenously’(Reference Jeejeebhoy, Matarese, Steiger and Seidner4). IF has been sub-classified into three types (Table 1) on the basis of duration and irreversibility(Reference Shaffer2, Reference Lal, Teubner and Shaffer3).
Table 1. Types of intestinal failure

Patients with Types 1 and 2 IF are expected to regain intestinal autonomy and can both be considered as Acute IF(5). Patients with Acute IF are usually found in surgical wards as these patients usually present on emergency surgical take or in a post-operative setting(5) The aims of this paper are to review the incidence, aetiology, prevention, management principles and outcome of acute IF.
Type 1 intestinal failure
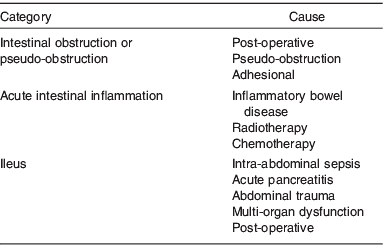
This is a common condition and is seen most frequently in a post-operative setting after abdominal surgery(Reference Lal, Teubner and Shaffer3) or in association with critical illnesses such as head injury, pneumonia, acute pancreatitis and post-cardiac surgery (Table 2)(Reference Pettigrew, Hill, Bouchier, Allan, Hodgson and Keighley6, Reference Carlson and Nightingale7). Post-operative ileus may occur in as many as 15% patients after intestinal resection(Reference Wolff, Viscusi and Delaney8). The patient should be investigated for underlying cause (electrolyte disturbance, pancreatitis and sepsis) which can be treated(5). The nutritional intervention for prolonged ileus is parenteral nutrition administered via a peripheral or central venous line.
Table 2. Categories and causes of Type 1 intestinal failure
Multimodal enhanced recovery techniques can reduce the frequency of ileus after abdominal surgery. These techniques allow administration of clear non-particulate oral fluids up to 2 h prior to induction of anaesthesia; avoid the use of mechanical bowel preparation, opiates and nasogastric tubes; aim to prevent Na and water overload; and promote early mobilisation and early introduction of diet(9).
These patients may need short-term administration of parenteral fluid and nutrition support before usually making a full recovery without complication. Such patients are usually managed in surgical wards, although some patients in critical care environments also fit into this category(10).
Type 2 intestinal failure
The incidence of Type 2 IF is unknown. The Department of Health has used a requirement for parenteral nutrition of 28 d or more as a surrogate marker of Type 2 IF. Using this definition, the annual incidence of Type 2 in England in 2008 was estimated to be nine patients per million population(10).
Type 2 IF is most often seen in the setting of an abdominal catastrophe(5). This may follow an acute event (mesenteric ischaemia, volvulus or trauma) necessitating massive enterectomy or occur as a complication of intestinal surgery (anastomotic leak; inadvertent and unrecognised intestinal injury) and result in enterocutaneous fistula or the need for resection and a proximal stoma(Reference Lal, Teubner and Shaffer3, 5, Reference Carlsson and Dark11). The most common underlying diagnoses are surgical complications (42%), Crohn's disease (21%) and mesenteric ischaemia (16%) followed by radiation enteritis, gastrointestinal dysmotility, trauma and malignancy(Reference Lal, Teubner and Shaffer3).
Aetiology and prevention of Type 2 intestinal failure
Post-operative complications
These include anastomotic leakage, intra-abdominal abscesses, intestinal obstruction (adhesions or volvulus) and post-operative intestinal ischaemia. In dealing with these complications, it may be necessary to resect bowel (leaving a permanently short bowel) and/or form a proximal stoma (temporary surgical disruption).
Thompson et al.(Reference Thompson, DiBaise and Iyer12) reported fifty-two patients with post-operative short-bowel syndrome (defined as small bowel length <180 cm) following operations such as colectomy, hysterectomy, appendicectomy and gastric bypass. The commonest causes of short bowel in that series were intestinal obstruction (adhesions or volvulus) and post-operative intestinal ischaemia. Patients undergoing resection for ischaemia or volvulus were more likely to have a remnant small bowel length <60 cm(Reference Thompson, DiBaise and Iyer12). Patients who develop short bowel after surgery for intestinal obstruction usually have had multiple operations(Reference Thompson, DiBaise and Iyer12).
Thompson et al.(Reference Thompson, DiBaise and Iyer12) proposed using strategies to prevent adhesions, avoiding technical errors and having a lower threshold for suspecting post-operative intestinal ischaemia. Patients with inflammatory bowel diseases (due to hypercoaguable state) and those undergoing prolonged laparoscopy (due to raised intra-abdominal pressure) are at increased risk of post-operative mesenteric ischaemia(Reference Thompson, DiBaise and Iyer12).
Crohn's disease
Short-bowel syndrome is not a frequent complication of surgery for Crohn's disease. Hurst et al.(Reference Hurst, Molinarir and Chung13) found that 5% of 464 patients with Crohn's disease undergoing operation had residual intestine less than 180 cm and seven patients required home parenteral nutrition. Post et al.(Reference Post, Herfarth and Bohm14) reported that short-bowel syndrome resulted in only one of 689 patients with Crohn's disease requiring operation. Where, short-bowel syndrome does complicate Crohn's disease, the most common reason is inadvertent injury of the intestine during laparotomy to deal with post-operative complications(Reference Agwunobi, Carlson and Anderson15).
With new strategies, such as strictureplasty and limited resection for the surgical management of Crohn's disease, it is expected that fewer patients will develop this complication(Reference Hurst, Molinarir and Chung13, Reference Fazio, Galandiuk and Jagelman16). In the hope of lessening the frequency and severity of short-bowel syndrome, the use of non-resectional options such as strictureplasty has become more common(Reference Gardiner and Dasari17). Patients with long-segment disease or multifocal disease would seem most likely to benefit from non-resectional strategies.
Mesenteric ischaemia
Acute mesenteric ischaemia can occur due to mesenteric vein thrombosis or arterial occlusion due to thrombosis or embolism. Small-bowel ischaemia may also occur due to volvulus and strangulated hernias(Reference Thompson, DiBaise and Iyer12).
In any patient with arterial or mesenteric thrombosis and bowel ischaemia the initial resection should be conservative and a second-look laparotomy should be planned to prevent resection of a potentially salvageable bowel.
Trauma
Trauma to the small bowel accounts for <1% of all trauma. Penetrating trauma accounts for 67% of small-bowel injuries, with blunt trauma accounting for 33%(Reference Dabney, Thompson and DiBaise18). Of those sustaining injury, 93% require intestinal resection. Intestinal resection after traumatic injury may be caused by trauma to mesenteric vessels and/or bowel wall(Reference Dabney, Thompson and DiBaise18). Direct bowel injury often results in multiple perforations but does not often lead to massive resections. Injuries to the superior mesenteric artery are rare and avulsion injuries of small mesenteric branches are a more common mechanism of injury and are associated with seat belt use(Reference Dabney, Thompson and DiBaise18). Areas of doubtful viability may be preserved and patients should undergo a second-look laparotomy to clearly delineate the need for resection. Appropriate resuscitation is important in preventing ongoing intestinal hypoperfusion and preventing the extension of the resection necessary for ischaemia.
However, trauma is responsible for <10% of patients with short-bowel syndrome(Reference Dabney, Thompson and DiBaise18).
Volvulus
This is a rare condition that can be associated with intestinal malrotation which can present with severe sudden abdominal pain. Unless operated on rapidly to correct the volvulus, it may progress to complete intestinal infarction with the need for sub-total enterectomy and formation of proximal jejunostomy. These patients usually have metabolic disturbances and sepsis in addition to the problems of a high-output proximal permanent stoma.
If at laparotomy, there are areas of doubtful viability, these areas should be preserved and a second-look laparotomy performed in 24 h to re-assess whether the ischaemic bowel is salvageable.
Radiation enteritis
Radiation enteritis can present with recurrent intestinal obstruction, fistulation or progressive weight loss(Reference Gidwani, Gardiner and Clarke19). The acute presentations are usually with intestinal obstruction. These patients are often undernourished, septic and have chronic small-bowel obstruction. The progressive weight loss and bowel obstruction can raise concern regarding recurrence of original malignancy (most often gynaecological). Most often it is the distal ileum that is affected by radiation injury and this can be resected without resulting in IF(Reference Gidwani, Gardiner and Clarke19). If the radiotherapy has been given post-hysterectomy or if para-aortic nodes have been included in the radiotherapy fields, a more extensive injury may result and surgery to relieve obstruction may result in acute IF.
Management of Type 2 intestinal failure
Patients with Type 2 IF are usually severely ill with systemic sepsis, metabolic disturbances and undernutrition(Reference Lal, Teubner and Shaffer3) and may have open wounds, stomas, fistulas and psychological upset. There is often pre-existing co-morbidity (IHD, diabetes mellitus, hypertension and chronic lung disease).
Management of Type 2 IF may be complex, prolonged, time consuming, expensive and daunting. There are a number of phases in the treatment and recovery of these patients, which have been summarised as the 4Rs: Resuscitation, Restitution, Reconstruction and Rehabilitation(Reference Kaushal and Carlson20). Successful treatment requires a multi-disciplinary team approach(Reference Lal, Teubner and Shaffer3) and can be greatly aided by a structured management approach(Reference Lal, Teubner and Shaffer3).
Such a structured approach has been described for the management of gastrointestinal fistulae. The group from Salford Royal NHS Trust(Reference Kaushal and Carlson20, Reference Chintapatla and Scott21) have described SNAP indicating that management of fistulae requires attention to sepsis and skin care (S), nutrition (N), anatomy definition (A) and planned procedure (P) for fistula closure in order of priority. Visschers et al.(Reference Visschers, Olde Damink and Winkens22) have described a similar approach (SOWATS) indicating that management of enterocutaneous fistulas consist of controlling Sepsis (S), Optimisation of nutritional care (O), Wound care (W), assessment of fistula anatomy (A), timing of surgery (T) and surgical strategy (S).
The principles of managing patients with acute IF have been described in the Association of Surgeons Great Britain and Ireland Guidelines on the Management of Patients with Acute Intestinal Failure(5) and can be summarised as SLOW STUFF: Sepsis (S), Liver dysfunction (L), Obstruction (O), Wound (W), Stoma (S), Talking (T), Undernutrition (U), Fluids and electrolytes (F) and Fistula (F). Most of these issues need to be dealt with during the early phases of Resuscitation and Restitution and many will require ongoing input throughout Rehabilitation and Reconstruction phases of the illness.
Sepsis
Sepsis may be characterised as usual by pyrexia, leucocytosis or raised inflammatory markers (C reactive protein) but may also be more insidious with hyponatraemia, liver dysfunction, jaundice or failure to make progress on nutrition support(5, Reference Kaushal and Carlson20, Reference Carlsson23). There should be a low threshold for investigation for occult sepsis by computerised tomography (CT) scan in patients with acute IF(Reference Gerzof and Oates24) as these patients require resuscitation and urgent elimination of sepsis by either percutaneous radiologically guided drainage or open surgery(Reference Kaushal and Carlson20). Drainage by CT guidance is effective in managing isolated collections but may be unsuccessful if a collection is fed directly by fistulating gut.
Open surgery is necessary if there are inaccessible or multiple intra-abdominal abscesses or a leaking anastomosis(Reference Kaushal and Carlson20). If the patient is unstable and hypoalbuminaemic, intestinal suture lines should not be left in continuity(Reference Kaushal and Carlson20). If there are enterotomies, these should be exteriorised or if repaired, they should be defunctioned proximally by a double-barrelled stoma(5). Drains may be placed and the abdominal cavity may be cleaned by lavage. If there is persistent abdominal infection, it may be appropriate to leave the abdomen open (laparostomy)(5, Reference Mughal, Bancewicz and Irving25).
Intra-abdominal sepsis due to enteric leakage will require either resection of the anastomosis and exteriorisation of the bowel ends, defunctioning of the anastomosis by raising a proximal loop jejunostomy or the creation of a laparostomy that leaves the abdomen open so that enteric contents can drain freely(Reference Chintapatla and Scott21). Patients with a laparostomy usually require to be nursed in an intensive care unit as they often have associated organ dysfunction.
Antibiotics are directed at expected causative organisms, and preferably chosen on the basis of culture results and choice may require close collaboration with microbiologists.
Failure to arrest intra-abdominal sepsis leads to multi-organ dysfunction/failure and results in ineffectiveness of nutrition support(Reference Lal, Teubner and Shaffer3); failure of fistula healing and ultimately patient death(Reference Soetors, Ebeid and Fischer26). Adequate management of sepsis is the most important factor that determines the outcome for patients with acute IF(5, Reference Carlsson23).
Liver dysfunction
Liver dysfunction is common in patients with acute IF and may relate to sepsis, administration of antibiotics or other drugs, biliary tract disease, cholestasis and overfeeding. Jaundice is most frequently associated with inadequately treated sepsis and requires investigation for sepsis by CT scanning.
Progressive liver dysfunction can result in hepatic fibrosis, portal hypertension and liver failure. Priorities are assessment for sepsis (CT scanning), biliary tract disease (ultrasound; consideration of ursodeoxycholic acid to improve bile excretion), review of medications and review of nutrition support (overfeeding; exploration of enteral feeding via nasogastric tube, stoma or fistula).
Obstruction
It may be difficult to distinguish between post-operative ileus and intestinal obstruction. If there is an onset of crampy abdominal pain and cessation of bowel action after initial opening of bowels in post-operative period, then mechanical obstruction is likely. Failure of water-soluble contrast to reach the colon within 4 h on CT scan is strongly predictive of failure of mechanical obstruction to resolve.
The vast majority of patients (>70%) with early post-operative intestinal obstruction will settle within 7 d on conservative treatment (nasogastric suction and nutrition support)(Reference Ellozy, Harris and Bauer27). Operation may be required if there is evidence of increasing abdominal tenderness, evidence of sepsis or failure to settle. Re-operation for intestinal obstruction in patients with IF is even more hazardous.
Wound
It may be necessary to leave the abdomen open (laparostomy) if there is extensive abdominal contamination (tertiary peritonitis) to facilitate the control of sepsis or there is a risk of compartment syndrome(5). If it is possible to safely close the abdomen, leaving it open confers no benefit and increases morbidity(Reference Robeldo, Luque-de-Leon and Suarez28). For other patients, wound infection and fascial dehiscence may result in an open wound or abdomen.
The combination of an open abdomen with an associated enteric fistula (together known as an enteroatmospheric fistula) is particularly difficult to manage and is associated with a much more considerable risk of death(Reference Rao, Burke and Finan29). A variety of techniques have been used to manage the open abdomen including topical negative pressure.
Topical negative pressure may be appropriate where the abdominal wall is intact, but should not be used in open abdominal wounds where intact loops of bowel are exposed, due to risk of inducing fistulation(Reference Rao, Burke and Finan29). There is currently a National Institute of Clinical Excellence audit of the management of open abdomens(30).
For open abdomens with associated fistula, wounds are ideally managed with a large Eakin bag with suction catheters placed through the bag to control the effluent and protect the skin(5). Management of patients with enteroatmospheric fistula is complex and should be referred to a specialist unit.
Stoma
The challenges with stomas in relation to acute IF are the output (high volume, highly irritant), position (created as an emergency and not optimally sited; in wound or in unusual site due to lack of choice) and body contours (previous scars, open wounds and presence of fistula). The consequences can be irritation of the skin, poor adherence of appliances to the skin, heavy stoma bags and frequent dislodgement of the stoma appliance.
Considerable skill and patience are required from the stoma and ward nurses to clean and dry the skin, filling in crevices with stoma paste, using high-output bags and additional reinforcing tapes to provide control of effluent and protection of skin.
Talking
It is essential that there is clear, consistent and well-documented communication between patients and their relatives. This can take multiple meetings, re-explanations and updates on progress to enable appropriate understanding of the range of problems, the priorities for action and the time frame in which recovery could be expected. Rather than talking about an estimated day of discharge, often the thinking is more in terms of the calendar and might refer to an estimated week or month of discharge. In addition to talking to the patient and their family, there needs to be communication with other disciplines (nutrition support, tissue viability, stoma team, pain team, physiotherapy, occupational therapists, psychologists, social worker, other medical disciplines – microbiology, radiology, gastroenterology, vascular surgeons).
This can be a very difficult time for the patient, family and the clinical team. The patient and their family often find it difficult to understand how the clinical condition has deteriorated to this extent and may persistently look for someone to ‘blame’.
Undernutrition
By definition, patients with acute IF require artificial nutritional support. They are unable to absorb sufficient orally administered energy (due to proximal fistula or stoma; or permanent short bowel) at a time when their metabolic demands are increased due to operation(s), procedures and sepsis.
Careful assessment of nutritional status and of requirements is necessary by the dietitian member of the Nutrition Support Team(5). The options for route of administration of nutrition support are peripheral or central parenteral nutrition or administration of enteral nutrition via the ileal mucous fistula (enteroclysis) or via the distal limb of the fistulous tract (fistuloclysis). Enteral feeding is safer and less expensive than parenteral nutrition but requires at least 75 cm of healthy small bowel distal to the fistula or stoma to offer any prospect of success (Reference Teubner, Morrison and Ravishankar31, Reference Levy, Frileux and Cugnenc32).
Parenteral nutrition is usually administered via a tunnelled central line(Reference Kaushal and Carlson20). It is vital that there is strict adherence to aseptic protocols to prevent line infections and to preserve venous access sites. Parenteral nutrition is also possible via peripheral veins but requires the use of lipid-containing, lower-osmolality solutions to reduce the incidence of thrombophlebitis(Reference Kaushal and Carlson20). Due to long-term high energy, N and fluid requirements this is rarely practical in patients with Type 2 IF.
Re-introduction of nutrition after a period of starvation carries the risk of development of re-feeding syndrome. It is therefore necessary to initially restore vitamin and minerals and correct any deficits of Mg, K or PO4; followed by introduction of the feed at a low rate with daily monitoring of fluid and electrolyte balance and blood glucose control. It can be difficult to assess response to nutritional interventions as changes in weight are more often due to fluid shifts and serum micronutrient concentrations are affected by sepsis(5).
Fluids and electrolyte balance
Fluid and electrolyte losses can be very high (4–6 litres/d) in patients with a proximal enterocutaneous fistula or proximal jejunostomy(Reference Kaushal and Carlson20). These losses can be particularly difficult to manage in patients with renal impairment. Meticulous records of all fluid losses are essential along with daily measurements of serum biochemistry and urinary Na concentration(Reference Kaushal and Carlson20).
Treatment is directed at limiting the oral intake of fluid (500–1000 ml/d), substituting rehydration solutions for normal oral fluids, reducing gastrointestinal secretory losses (proton pump inhibitor, octreotide), slowing intestinal transit (loperamide, codeine) and replacing fluid and electrolytes (directed by fluid balance charts and measures of serum and urinary chemistry)(Reference Kaushal and Carlson20).
Fistula
Enterocutaneous fistulas can be associated with sepsis (requiring assessment by CT scan to identify and drain associated abscesses), high fluid and electrolyte losses (mentioned earlier) and skin irritation. The fistulas often occur at awkward sites (through old wounds), in association with contour deformity and it can be very difficult to get a satisfactory collection of the fistula effluent. Fistula effluent can cause chemical irritation of the skin and pain. Repeated leaks from appliances can limit patient mobility and rehabilitation as well as being psychologically very distressing(5, Reference Kaushal and Carlson20). Stoma nurses and/or tissue viability nurses can be very helpful in finding a solution to protect the skin that can involve the use of low-grade suction catheter placed through a stoma appliance(Reference Hughes, Myers, Carlson and Nightingale33).
It is sometimes necessary to perform a defunctioning loop jejunostomy in the left upper-abdominal quadrant to divert enteric contents from the fistula(Reference Carlson and Nightingale7, Reference Carlsson23).
Definitive surgery for non-healing enterocutaneous fistulas requires elimination of sepsis (Reference Visschers, Olde Damink and Winkens22), restoration of nutrition, delineation of anatomy (by oral and per-fistula contrast studies) and a planned procedure after a period of 3–6 months(Reference Scripcariu, Carlson and Bancewicz34) or when there is evidence of reduction of adhesions (softening of abdominal wall, development of hernia or prolapse of fistula)(Reference Kaushal and Carlson20).
Although recovery of these patients takes a long time (SLOW STUFF) and some interventions are slow to have an effect (restoration of nutrition, management of open abdomen, recovery of disturbed liver function), other actions of the clinical team need to be taken promptly (correction of fluid and electrolyte abnormalities; elimination of sepsis).
Resources and acute intestinal failure
The decision about whether a surgical unit can manage a patient with acute IF is complex and will depend on whether the unit has the necessary expertise and resources (including the ability to share the burden with colleagues), intact relationships with patient and family and acceptable outcomes for the management of such patients.
The resources needed to manage patients with acute IF have been described in the Association of Surgeons of Great Britain and Ireland Guidelines on the Surgical Management of Patients with Acute IF(5) and detail the need for:
a nominated lead surgeon (trained in gastrointestinal and IF surgery, a member of an appropriate specialist association, attached to a nutrition support team, attending continuing medical education in IF and nutrition support; and supported by surgical colleagues),
a surgical unit with nursing expertise in gastrointestinal surgery, senior surgical trainees, regular morbidity and mortality meetings, audit programme and research interest,
a fully functional nutrition support team and
a hospital with an 24 h/d emergency theatre, a gastrointestinal theatre team, critical care facilities, acute pain team, imaging department with expertise in interventional radiology and cross-sectional imaging, stomatherapy and tissue viability nursing teams, access to other surgical teams (urological, plastics and gynaecological) and microbiology expertise.
When to transfer a patient with acute intestinal failure
The National Specialised Commissioning Advisory Group(35) has recognised the difficulty in managing patients with acute IF and have funded two National Intestinal Failure Units (St Mark's, Northwick Park and Salford Royal NHS Trust). Criteria have been defined(5) to indicate the type of patients that should be referred to these National Units and have been summarised in Table 3.
Table 3. Criteria for admission to National Intestinal Failure Units

Most of the time, patients are transferred because their problems exceed the capability of a unit (from medical, psychological or communication reasons) to continue providing care for them.
A decision is necessary as to whether the patient is likely to benefit from the transfer. This decision may be aided by the provision of accurate verbal and written information, by a visit to the sending unit or by transferring the patient for assessment. Transfer for assessment needs to be discussed clearly with the patient and his family to prevent unrealistic expectations in the event that the patient needs to return to original unit.
How not to transfer a patient with acute intestinal failure
Patients with acute IF can sometimes be transferred with one or more volumes of incomplete loose-leaf variety hospital notes, late in the evening (due to difficulty with access to beds or difficulty arranging transport), accompanied by a hand-written, barely legible transfer letter written by a junior medical staff member and by relatives who have poor understanding of the reasons for transfer or the unrealistic expectation that their relative is going to be discharged a new person in 3 d time.
It can be extremely difficult for the receiving unit to find their way through unfamiliar notes, discover all the relevant details of the procedures performed and the results of investigations completed. It can be difficult in retrospect to understand why decisions were made and what explanations have been made to the patient and his family.
How to transfer a patient with acute intestinal failure
Contact with the receiving hospital should be consultant-to-consultant with a clear identification of the reason(s) for transfer and a step-by-step sequence of events leading up to the transfer.
It is helpful if detailed information about the patient is provided in advance of the transfer by a senior doctor describing the procedures carried out in time order, findings at operations and operative difficulties encountered; co-morbidity; presence of resistant organisms; description of wounds, stomas or fistulas; active treatments; recent blood microbiological culture and pathology results; fluid balance and nutrition interventions; and the presence of lines and tubes.
The patient should be transferred with a written referral letter, all notes and scans (if it is not possible to view these by web link), up-to-date blood results, accurate fluid balance and drug prescription and with a member of clinical team who can provide a safe and comprehensive handover. It is helpful if the referring consultant can provide a contact number to deal with queries.
Receiving a patient with acute intestinal failure
The receiving unit needs to be clear about what information is required (referral letter, all notes and scans, most recent chemistry; details of drugs, fluid balance and infective status). It is useful for ward nursing staff to obtain details concerning wound problems and current management, mobility and family contact details prior to transfer. The nutrition support team and/or pharmacist can obtain information regarding provision of parenteral nutrition and fluids from the pharmacy in the sending hospital.
It can be helpful for the receiving unit clinician to visit the patient in the transferring unit depending on the geographical distance between units. This offers the opportunity to see the problems first hand and to permit direct discussion with clinical team, patient and relatives.
Outcome
Patients with acute IF can have an in-hospital mortality as high as 13%(Reference Scott, Leinhardt and O'Hanrahan36). Visschers et al.(Reference Visschers, Olde Damink and Winkens22) report a mortality rate of 9·6% for management of patients with enterocutaneous fistula. Post-operative morbidity includes recurrent intestinal obstruction, abscesses, fistula, wound infections, incisional hernias and long-term need for parenteral nutrition(Reference Harris, Kelly and Pockaj37).
In addition to mortality, the Association of Surgeons Guidelines on the management of patients with acute IF(5) have suggested that quality measures for managing patients with acute IF include infection rate in central lines, the rate of unplanned return to theatre, the recurrent fistula rate and the success in discontinuation of artificial nutrition support (after restorative surgery) and the prevention of Type 3 IF and the need for long-term parenteral nutrition (Reference Lal, Teubner and Shaffer3, Reference Carlsson23).
Surgical reconstruction of patients with IF can be very challenging with difficulties in entering the abdominal cavity, dissecting apart dense adhesions without causing enterotomies, dealing with intra-abdominal abscess cavities, performing anastomoses and closing abdomens (e.g. component separation techniques)(5, Reference Kaushal and Carlson20). This Complex Re-Operative Surgery for Intestinal Failure is therefore delayed for at least 3–6 months until sepsis and acute inflammation are resolved, undernutrition corrected, the patient mobilised (rehabilitation phase), anatomy delineated (contrast studies and cross-sectional imaging) and adhesions lessened (soft abdominal wall, prolapsing stoma or fistula; herniating wound). Re-operation in patients with a frozen abdomen results in intestinal resection in 90% patients(Reference Harris, Kelly and Pockaj37). Inadvertent enterotomy during re-laparotomy is strongly predictive of post-operative complications(Reference Van der Krabben, Dijkstra and Nieuwenhuijzen38). The general principles of reconstructive surgery for IF have been described in the Association of Surgeons Guidelines for the Management of patients with acute IF(5).
Conclusions
In recent years, there has been considerable progress in the definition of IF and in its sub-classification (Types 1–3). It has been difficult to define the incidence and prevalence of acute IF although an estimated annual incidence of nine patients/million population in England has now been calculated using a surrogate marker (need for parenteral nutrition for 28 d or more). The aetiology of acute IF is most commonly due to post-operative complications, Crohn's disease, mesenteric ischaemia, trauma, volvulus and radiation enteritis. There is now general agreement that the management of acute IF can be aided by the involvement of a multi-disciplinary team and using a structured management approach (SNAP and SOWATS). The resources needed to manage acute IF and clinical indicators of quality of care are now being defined. The indications for referral of patients to a National Intestinal Failure Unit have been published(35) and practical advice on how to transfer a patient with acute IF has been provided in this paper.
Acknowledgement
The author declares no conflicts of interest.





