Regular physical activity and exercise has been associated with a number of health benefits including reduced risk of developing CHD, stroke, type 2 diabetes and some forms of cancer. Mechanistically, these effects are mediated through improvements in numerous risk factors for disease such as blood pressure, lipoprotein profile, inflammation, insulin sensitivity and weight management(Reference Garber, Blissmer and Deschenes1). Regular physical activity and exercise has also been increasingly recognised for its therapeutic potential in many clinical contexts such as obesity, type 2 diabetes and CVD(Reference Colberg, Sigal and Yardley2, Reference Ross, Blair and Arena3). Indeed, the concept of exercise as medicine has gained significant traction in recent years with initiatives such as Exercise is Medicine® (http://www.exerciseismedicine.org/) managed by the American College of Sports Medicine established to increase the use of exercise programmes within primary and other health care settings. A parallel exists between the salutary effects of exercise and the clinical effectiveness of many other medications in that some individuals display a less than expected therapeutic response. For example, the HERITAGE Family Study showed that structured regular aerobic exercise training led to increased insulin sensitivity (determined by intravenous glucose tolerance test) on average in previously sedentary individuals. However, of the entire cohort, 42 % of participants displayed no change or decreased insulin sensitivity(Reference Boulé, Weisnagel and Lakka4). A further study reported that 12-week structured aerobic exercise training resulted in weight loss of about 4 kg on average, although over 50 % of participants were identified as losing less weight than predicted(Reference King, Hopkins and Caudwell5). Whether such observations reflect true inter-individual variability in responsiveness to exercise training is debated, but the evidence does indicate that some individuals may not be achieving the full potential benefits of exercise. Physical activity relates to any type of movement that requires muscle contraction and raises energy expenditure, a sub-component of which is structured, volitional exercise. While generic physical activity and exercise guidelines are clearly established, identifying strategies to maximise the therapeutic benefit for all individuals represents an important step in the refinement and optimisation of public health physical activity recommendations.
Sport and exercise scientists have been studying nutrient–exercise interactions for decades in the search for nutritional strategies that may contribute to improving exercise performance. It is well known that nutrient intake around exercise can interact with, and modulate, metabolic, hormonal and molecular responses that may ultimately influence exercise adaptation in endurance-trained individuals(Reference Hawley John, Maughan Ronald and Hargreaves6). While ensuring a high dietary carbohydrate intake remains critical for optimising acute endurance exercise performance and recovery, such a strategy has been proposed to blunt some of the key skeletal muscle adaptive responses to exercise training(Reference Bartlett, Hawley and Morton7). Accordingly, endurance athletes are now advised to consider a periodised dietary approach by altering nutrient, and particularly carbohydrate intake, as appropriate to support their training and performance goals(Reference Burke, Hawley and Wong8). This may include undertaking selected training sessions under conditions of low carbohydrate availability in order to maximise the adaptive response to exercise, such as performing exercise in the overnight-fasted v. postprandial state. Much of the nutrient–exercise interaction research has occurred in the sports performance domain, but there is an increasing interest in exploring its potential translation into optimising exercise responses for health or therapeutic benefit(Reference Hansen, De Strijcker and Calders9, Reference Haxhi, Scotto di Palumbo and Sacchetti10). It is well known that the effectiveness of some medicines may be reduced by the presence of nutrients in the gastrointestinal tract or the direct effects of nutrients on drug metabolism(Reference Genser11). An important question in light of maximising the therapeutic benefit of exercise for all individuals is whether exercise, such as some medicines, is best taken on an empty stomach? Perhaps the less than expected response to exercise training observed in some individuals relates to their feeding status about individual exercise bouts? Accordingly, in this narrative review, we evaluate the impact of undertaking aerobic exercise in the overnight-fasted v. fed-state in the context of optimising the health or therapeutic benefits of regular physical activity.
Short-term metabolic responses to overnight-fasted v. fed-state exercise
Energy production during sustained aerobic exercise performed in the overnight-fasted state (i.e. 8–12 h) is supported primarily by the oxidation of endogenous fat and carbohydrate stores. Fat oxidation predominates during low-intensity exercise (<45 % VO2max), both fat and carbohydrate oxidation increase to support moderate-intensity exercise (45–65 % VO2max), while under most conditions carbohydrate oxidation predominates at higher exercise intensities (>65 % VO2max). A recent systematic review and meta-analysis clearly demonstrated that as compared with performing exercise in the overnight fasted-state, the consumption of a carbohydrate-containing meal between 0·5 and 3 h before exercise reduces fat oxidation (and increases carbohydrate oxidation) during exercise performed for up to 2 h duration at <70 % VO2max (Reference Vieira, Costa and Macedo12). The suppression of fat oxidation during fed-state exercise occurred regardless of exercise duration, participant sex, BMI, exercise training status, duration between feeding and exercise or meal carbohydrate content. Plasma NEFA concentrations did not significantly differ between exercise performed in the fed v. overnight-fasted state. A clear effect of fed-state exercise on blood glucose and insulin concentrations points towards increases in glycolytic flux as the dominant regulator of fuel metabolism in these conditions(Reference Coyle, Jeukendrup and Wagenmakers13). Further, previous research has established that the increased fat oxidation observed during exercise in the overnight-fasted state appears to be supported by both increased plasma long-chain fatty acid oxidation and type 1 skeletal muscle fibre intramuscular TAG utilisation at least in lean individuals(Reference Coyle, Jeukendrup and Wagenmakers13, Reference De Bock, Richter Erik and Russell Aaron14). There does not appear to be any modulation of liver fat during exercise regardless of whether this was performed in the overnight-fasted or fed-state(Reference Bilet, Brouwers and van Ewijk15).
Whether the increased fat oxidation when exercise is performed in the overnight-fasted state can impact upon daily fat oxidation, which would be more representative of long-term potential to alter fat balance, is of paramount importance. Traditionally, despite exercise increasing fat oxidation during the exercise bout itself, increases in fat oxidation and reductions in fat balance over a 24 h period measured using whole-room indirect calorimetry have not been observed when studied under conditions of energy balance(Reference Melanson, Gozansky and Barry16). This has been attributed to the effects of insulin as a consequence of consuming carbohydrate-containing meals suppressing lipid utilisation throughout the day. However, it is notable that exercise in these studies was not undertaken in the overnight-fasted state. Iwayama et al. demonstrated recently in lean healthy men that 1 h moderate-intensity exercise results in increased 24 h fat oxidation measured using whole-room indirect calorimetry when exercise was performed before (i.e. in the overnight-fasted state) breakfast, but not after lunch or dinner, even when participants remained in overall energy balance(Reference Iwayama, Kurihara and Nabekura17). The same group have also shown improved 24 h fat oxidation and fat balance with pre-breakfast exercise in women(Reference Iwayama, Kawabuchi and Nabekura18). Exercise performed in the overnight-fasted state would appear necessary to alter 24 h fat oxidation. While this area has not received extensive mechanistic investigation, negative correlations between energy balance or carbohydrate balance with 24 h fat oxidation suggests transient energy and/or carbohydrate deficits may be driving the response(Reference Iwayama, Kurihara and Nabekura17).
The study of nutrient–exercise interactions in the context of substrate oxidation is important because links between fat oxidation during exercise and daily fat oxidation have been made(Reference Robinson, Hattersley and Frost19), as have associations between daily fat oxidation and obesity risk(Reference Zurlo, Lillioja and Puente20). Of potentially equal importance is consideration of how timing of food intake around exercise modulates other risk factors for cardio-metabolic diseases, such as circulating lipid and glucose concentrations. With regard to blood lipid profiles, a study by Enevoldsen et al. is insightful(Reference Enevoldsen, Simonsen and Macdonald21). This group determined blood metabolite and hormone responses across the course of 5·5 h in young healthy men who undertook exercise either before or after mixed macronutrient meal ingestion. Over the duration of the study period, a more favourable response of circulating markers of lipid availability (e.g. lower plasma TAG and VLDL-TAG concentrations) was observed when exercise was performed before as compared to after meal ingestion. A similar result was observed more recently in a study of overweight men, whereby exercise followed by food intake but not food intake followed by exercise significantly lowered plasma TAG concentrations as compared with a non-exercise control trial(Reference Farah and Gill22). Collectively, the afore-mentioned studies imply a potentially beneficial impact of overnight-fasted v. fed-state exercise on the aspects of body fat regulation and lipid metabolism at least in the context of single bouts of exercise.
The impact of overnight-fasted v. fed-state exercise on the aspects of glycaemic control has been subject to considerable recent debate(Reference Chacko23). Provision of carbohydrate-containing meals increases blood glucose and insulin concentrations, but when this is followed by exercise glucose uptake into skeletal muscle is enhanced leading to a lowering effect on blood glucose. This has been argued to be of particular importance in the context of diabetes, with the commencement of exercise 30–90 min post-prandial suggested to be optimal in accelerating meal-derived glucose disposal thus avoiding hyperglycaemia but also minimising risk of post-exercise hypoglycaemia(Reference Chacko23). This glucose-lowering effect of fed-state exercise in type 2 diabetes does appear to be most pronounced in those with the highest pre-exercise blood glucose concentrations(Reference Poirier, Tremblay and Catellier24). Taking subsequent meals into account can present a different picture. For example, feeding prior to exercise in lean, healthy individuals has been observed to increase the post-exercise postprandial glucose response to mixed macronutrient ingestion as compared with fasted-state exercise(Reference Gonzalez25). Furthermore, when glycaemic control was assessed in individuals with prediabetes over the course of a full day after overnight-fasted or fed-state exercise using continuous glucose monitoring, interstitial glucose variability but not total interstitial glucose exposure (area under the curve) was improved with fed-state exercise(Reference Nygaard, Rønnestad and Hammarström26). It would seem that the reported influence of a single bout of overnight-fasted v. fed-state exercise on short-term glycaemic control can be affected by the experimental conditions (e.g. when assessed over single v. multiple meals), the time frame of measurement and possibly the populations studied. However, as will be discussed in a later section, it is critically important to consider the difference between the acute effects of a single exercise bout and the adaptive response to chronic exercise training resulting from the culmination of those single exercise bouts.
Short-term energy balance behaviour responses to overnight-fasted v. fed-state exercise
Exercising in the overnight-fasted v. fed-state will clearly lead to a longer period of energy deficit, and from an energy balance perspective, it is important to understand the extent to which compensation of this energy deficit may occur during the post-exercise period.
This was initially investigated in a study by Gonzalez et al. who had twelve young physically active men undertake 1 h moderate-intensity treadmill running exercise performed in the overnight-fasted state or 2 h after breakfast consumption(Reference Gonzalez, Veasey and Rumbold27). After exercise, all participants consumed a standardised mixed-macronutrient drink, followed 90 min later by provision of an ad libitum test lunch, allowing for calculation of energy and macronutrient intake. Indirect calorimetry was conducted during the experiment to calculate energy expenditure and substrate oxidation. This group reported that despite the absence of breakfast in the overnight-fasted exercise, energy intake during the test lunch was similar when exercise was performed in the fed-state. Accordingly, energy intake and energy balance across the entire study period was significantly less when exercise was performed in the overnight-fasted v. fed-state. Interestingly, the lower energy balance with overnight-fasted exercise was attributable to reduced fat but not carbohydrate balance, the importance of which are 2-fold. First, greater reductions in fat balance may be more likely to induce favourable effects on body fat loss if sustained over time. Secondly, it is possible that the maintenance of carbohydrate balance is more important and tightly regulated than fat balance possibly due to finite carbohydrate storage capacity as has been previously suggested(Reference Jean-Pierre28). In support of this assertion, it has been reported that individuals who utilise more carbohydrate during exercise are more likely to compensate for the energy expended during exercise with greater post-exercise energy intake(Reference Hopkins, Blundell and King29), and mice overexpressing hepatic protein targeted to glycogen (resulting in increased liver glycogen concentrations under fasted and fed conditions) display reduced energy intake and increased energy expenditure(Reference López-Soldado, Fuentes-Romero and Duran30). As such, interventions that minimise carbohydrate oxidation during exercise (such as overnight-fasted exercise) may serve to limit subsequent energy intake.
More recently, the effects of overnight-fasted v. fed-state exercise (i.e. breakfast) on energy intake was examined over the course of an entire day, which may be more reflective of the potential for long-term impact on energy balance(Reference Bachman, Deitrick and Hillman31). The study by Bachman et al. reported that ad libitum food (and energy) intake over a 24 h period was lower when exercise was performed prior to breakfast consumption. Interestingly, reduced energy intake was not simply a function of breakfast omission but food intake during meals and snacks consumed later in the day suggesting more prolonged effects of overnight-fasted exercise on regulation of food intake. At this stage, the involvement of metabolic (e.g. carbohydrate status) and/or modulation of appetite hormone regulatory mechanisms in explaining this lower energy intake with overnight-fasted exercise has not been resolved. The possibility of simply having less time in the day to consume food should also not be excluded, and while speculative, if this is relevant then aspects of the purported benefits of time-restricted feeding may be worthy for consideration(Reference Hutchison, Wittert and Heilbronn32). Importantly, the study by Bachman et al. did not quantify energy expenditure across the entire study period, thus the overall impact of the interventions on energy balance was not reported. This may be particularly important, as in conditions where free-living expenditure has been quantified, omission of breakfast per se (i.e. without exercise intervention) may transiently lower physical activity energy expenditure which would impact on energy balance(Reference Betts, Chowdhury and Gonzalez33). These collective studies suggest that short-term studies that encompass and allow for the behavioural responses to overnight-fasted v. fed-state exercise to occur may be particularly useful for understanding the potential for long-term impacts upon metabolism and health outcomes. However, the impact of altering carbohydrate oxidation on all components of energy balance (including physical activity) in the post-exercise period currently remains unknown, as do the mechanisms that link carbohydrate balance to any behavioural responses in human subjects.
Longer term metabolic and health outcomes in response to overnight-fasted and fed-state exercise
It is clear from the previous sections that overnight-fasted v. fed-state exercise can modulate metabolic and behavioural responses to a single bout of exercise. A relevant question is the extent to which such short-term responses translate into long-term modifications in biomarkers or risk factors for cardio-metabolic disease. If the feeding status around single exercise bouts is influential in determining long-term adaptive responses to exercise, then it may in part explain why some individuals do not always adapt to exercise training as would be predicted. The implication would be that if all exercise training sessions within a training study were undertaken with standardisation of pre-exercise nutrition, adaptive responses may be more consistent. However, to our knowledge, there are no studies investigating overnight-fasted v. fed-state exercise training on the consistency or variability of exercise adaptation. Indeed, with a few exceptions described below, the vast majority of aerobic exercise training intervention studies focus on the exercise component rather consideration of nutritional control or timing of food intake around exercise bouts.
The effects of overnight-fasted v. fed-state exercise on total body mass and indices of body composition have been investigated in training studies conducted under differing states of energy balance. Under isoenergetic and hypoenergetic conditions, when the state of energy balance is matched between intervention groups, responses of total body mass, fat mass or fat-free mass did not differ as a function of short-term (i.e. 4–6 weeks) overnight-fasted v. fed-state exercise training(Reference De Bock, Richter Erik and Russell Aaron14, Reference Gillen, Percival and Alison34–Reference Schoenfeld, Aragon and Wilborn36). While the effects on total body mass may be predictable, the lack of difference in body fat reduction contrasts what could theoretically be expected based on previously observed increases in daily fat oxidation and less positive (more negative) fat balance as a result of conducting overnight-fasted exercise in acute studies. One of the afore-mentioned studies utilised a high-intensity interval training programme(Reference Gillen, Percival and Alison34), which may not be favourable for increasing fat oxidation during exercise. As well, in all studies, the duration of training (i.e. 4–6 weeks) may have been insufficient to realise the theoretical advantages of overnight-fasted exercise training on body fat mass. Previous studies of exercise training per se would indicate that at least 12 weeks is necessary to induce measurable reductions in body fat(Reference Ross, Dagnone and Jones37, Reference Ross, Janssen and Dawson38). Thus, to date, experimental conditions may not have been optimised to conclusively study if body composition can be improved with regular overnight-fasted v. fed-state exercise training in iso and hypoenergetic conditions. Indeed it could be that any short-term changes in daily substrate oxidation and storage are balanced out over periods of days and weeks such that body composition remains unaltered over the long term unless there are clear perturbations to long-term energy balance(Reference Hall and Guo39).
In contrast, a study conducted by Van Proeyen and colleagues indicated that effects of overnight-fasted exercise training on body composition may be revealed during conditions of hyperenergetic feeding(Reference Karen, Karolina and Henri40). These researchers subjected three groups of lean, healthy men to 6 weeks of 30 % excess of habitual energy intake in the form of a fat-rich (50 % dietary energy) diet. Participants either performed no exercise (control, Con), overnight-fasted (Fast) or fed-state (Fed) exercise four times per week. In Con and Fed, body mass significantly increased as compared with pre-diet values by about 3 and 1·4 kg, respectively, while no significant changes were observed in Fast. While interesting, it should be noted that despite apparent within-group differences in body mass gain, no significant between-group differences were observed between Fed and Fast. Incidentally, body fat assessed using skinfold thickness measurements increased in Con, but did not change significantly in Fed or Fast. Overall, there is a paucity of evidence to support a clear influence of overnight-fasted v. fed-state exercise training on body weight and composition, at least when studied over a short duration of training and in the state of energy balance is matched between intervention arms. However, as we discuss later, a more fruitful approach in the context of body weight and composition may be to not clamp energy balance between interventions and allow natural alterations in energy balance behaviours to occur outside of the specific controlled fasted or fed-exercise prescription.
Exercising in the overnight-fasted v. fed-state has been linked to a number of responses that could plausibly translate to long-term improvements in lipid and glucose metabolism. Adipose tissue plays a critical role in the storage of ingested dietary fats with relevance for post-prandial lipemia and minimising ectopic lipid storage. Indeed, high turnover of adipose tissue lipid stores has been associated with improved metabolic health(Reference Arner, Bernard and Salehpour41, Reference Frayn, Bernard and Spalding42) suggesting that frequent oxidation of adipose tissue fatty acids increases the ability of adipose tissue to buffer lipid flux. Feeding status may therefore alter adipose tissue physiology with resultant implications for health. Consistent with this line of reasoning, a single bout of fed-state exercise blunts the effects typically seen with overnight-fasted exercise on the expression of genes related to lipid metabolism, insulin sensitivity and glucose uptake in adipose tissue(Reference Chen, Travers and Walhin43). In a similar manner, fed-state exercise tends to blunt acute exercise-related responses in skeletal muscle molecular pathways associated with the up-regulation of oxidative, lipid and carbohydrate metabolism (e.g. gene expression of FAT/CD36, CPT1, UCP3, PDK4, GLUT4, AMPKα2)(Reference Civitarese, Hesselink and Russell44, Reference Cluberton, McGee and Murphy45). As well, in young lean men, overnight-fasted but not fed-state exercise increased utilisation of intramuscular TAG in type 1 skeletal muscle fibres(Reference De Bock, Richter Erik and Russell Aaron14); high rates of intramuscular TAG turnover (i.e. storage and breakdown for NEFA oxidation) have been implicated in the maintenance of muscle insulin sensitivity(Reference Shaw, Clark and Wagenmakers46). Finally, while not unequivocal(Reference Proeyen, Szlufcik and Nielens35, Reference Bock, Derave and Eijnde47), greater long-term changes in markers of skeletal muscle training adaptation such as the protein contents of GLUT4, FAT/CD36 and FABP and the maximal activities of the mitochondrial enzymes citrate synthase and β-hydroxyacyl-coA dehydrogenase have been observed with overnight-fasted exercise training(Reference Proeyen, Szlufcik and Nielens35, Reference Karen, Karolina and Henri40).
In general, the afore-mentioned evidence points to the potential for overnight-fasted exercise to promote greater benefits to metabolic health outcomes than conducting regular exercise in the fed-state, although the number of investigations in this area is remarkably limited. Summarising the evidence that is available to date, Hansen et al. concluded that there does not appear to be clear impact of short-term fed v. fasted-stated exercise training on overnight-fasted resting blood markers such as glucose, insulin and NEFA; notably studies have generally been performed in young lean individuals(Reference Hansen, De Strijcker and Calders9). Only the study by Van Proeyen and colleagues described previously, which adopted 6 weeks of hyperenergetic fat-rich feeding, has addressed the impact of overnight-fasted v. fed-state exercise training on a dynamic measure of metabolic function(Reference Karen, Karolina and Henri40). These authors found that the Matsuda Insulin Sensitivity Index (calculated from an oral glucose tolerance test) was higher in the group that performed overnight-fasted exercise training as compared with the no exercise control trial. No significant differences were reported between fed-state exercise and no exercise control, with the implication that fasted-state exercise improves glucose tolerance during a fat-rich diet. The authors rightly acknowledge that body mass gain in the control (and fed-exercise) trial but not the fasted-exercise trial could contribute to the observed differential responses to insulin sensitivity. Nonetheless, these data provide promising proof of concept for a role for overnight-fasted training in enhancing benefits of exercise on glucose tolerance at least under conditions of excess energy intake, which could have relevance within obesogenic environments.
As stated earlier, there is a clinical view that post-prandial exercise is preferable over fasted-state exercise for the acute control of blood glucose, at least in patients living with type 2 diabetes(Reference Chacko23). However, if an individual performs fasted-state exercise, it is not clear if the subsequent post-prandial rises in blood glucose are pathological. As well, we also highlighted earlier that as compared with fasted-state exercise, fed-state exercise can result in a worsening of subsequent post-prandial glucose control(Reference Gonzalez25). Again, the question arises as to whether this is potentially pathological or simply reflecting short-term adaptive physiology. Our recent work examining post-prandial glucose fluxes after no exercise, exercise performed in the overnight-fasted or fed-state would suggest the latter(Reference Edinburgh, Hengist and Smith48). Specifically, we observed in healthy young men that fed-state exercise increases glucose appearance rates into the circulation during subsequent glucose ingestion, and this was explained by increases in the appearance of the ingested glucose. However, this was met with increases in whole body glucose disposal, such that the increased influx of glucose was appropriately cleared. Whether this applies in other study populations remains to be determined, but it does lend support to the notion that the responses of blood glucose to single bouts of exercise performed in the fed or fasted state are part of normal physiology. What is perhaps more important is the adaptive stimulus provided by acute bouts of exercise, for example, in skeletal muscle, that when accrued over time results in chronic changes in the capacity to manage postprandial excursions in blood glucose (and lipids). In this respect, the work from Van Proeyen and colleagues showing that the aspects of glucose control may be preferentially affected by consistent exercise training in the overnight-fasted v. fed-state (under conditions of excess energy intake) is perhaps most revealing(Reference Karen, Karolina and Henri40), although clearly there is a need to follow-up this work in patients at risk of or living with disturbances in glucose control such as type 2 diabetes.
Conclusions
There is little doubt that the investigation of how nutrient intake in and around exercise might modulate the metabolic, molecular and adaptive responses to exercise training is of major current interest(Reference Hansen, De Strijcker and Calders9, Reference Haxhi, Scotto di Palumbo and Sacchetti10, Reference Chacko23, Reference Hawley, Lundby and Cotter49–Reference Solomon, Eves and Laye51). However, there is a need for further research in order to fully elucidate if overnight-fasted exercise could be a means to optimise the health benefits of physical activity. For example, the influence of a single bout of overnight-fasted v. fed-state exercise on the aspects of lipid metabolism and the molecular signals underpinning training adaptation should be studied further in populations at risk for cardio-metabolic disease. Characterising 24 h profiles of circulating metabolite and hormones related to glucose and lipid metabolism in participant populations across the health continuum would help to clarify their modulation of overnight-fasted or fed-state exercise. As well, there is a need to characterise the influence of overnight-fasted or fed-state exercise on short-term energy balance behaviours in a range of study populations, as this could more adequately reflect responses in real-world settings. Generation of these data could provide clearer insights into which populations and outcome measures may yield greater benefits from long-term exercise training in overnight-fasted conditions.
There is also a need to extend exercise training studies performed in the overnight-fasted state v. fed-state for longer durations (i.e. ≥12 weeks) and into population groups with or at risk for cardio-metabolic disease. In doing this, it would be important to integrate important clinical outcomes such as body mass and composition, glucose tolerance, glycated Hb and lipid profiles with measures of whole-body and tissue-specific metabolic function in order to gain further mechanistic insights (e.g. hepatic, adipose and skeletal muscle adaptation). Given that daily variations in glycaemic and lipid profiles could impact upon the aspects of vascular function (e.g. endothelial function, microvascular perfusion), this would also be an important area to explore. In conducting exercise training studies, an important consideration is whether or not to match the state of energy balance between intervention groups. As there appears to be little compensation of energy intake to acute bouts of overnight-fasted exercise, this approach appears most likely to produce more consistent reductions in energy balance, which over the long term may provide complementary benefits to many outcome measures relevant to metabolic health. Mechanistically, it is always appealing to tease out intervention effects independent from changes in body mass(Reference Zarins, Wallis and Faghihnia52). However, if additional health benefits are to be gained from overnight-fasted v. fed-state exercise, it is probably a moot point as to whether the effects arise through direct or indirect mechanisms related to the intervention. Finally, while the focus of the present review was on aerobic exercise, future work investigating the health impact of performing other forms of exercise such as resistance training(Reference Frawley, Greenwald and Rogers53) or combined resistance and aerobic training (concurrent exercise) in the overnight-fasted v. fed-state would be worthwhile.
In conclusion, conducting aerobic exercise in the overnight-fasted v. fed-sate can differentially modulate the aspects of metabolism (Fig. 1) and it is possible that this could influence the overall adaptive response to exercise training for health benefits. If this is the case, advice on when to exercise with respect to food intake could be considered for incorporation into physical activity guidelines in general or for specific sub-populations seeking to optimise the health or therapeutic benefits of exercise. However, further research, some of which is highlighted in this review, is needed before we can answer the question as to whether exercise is best served on an empty stomach.
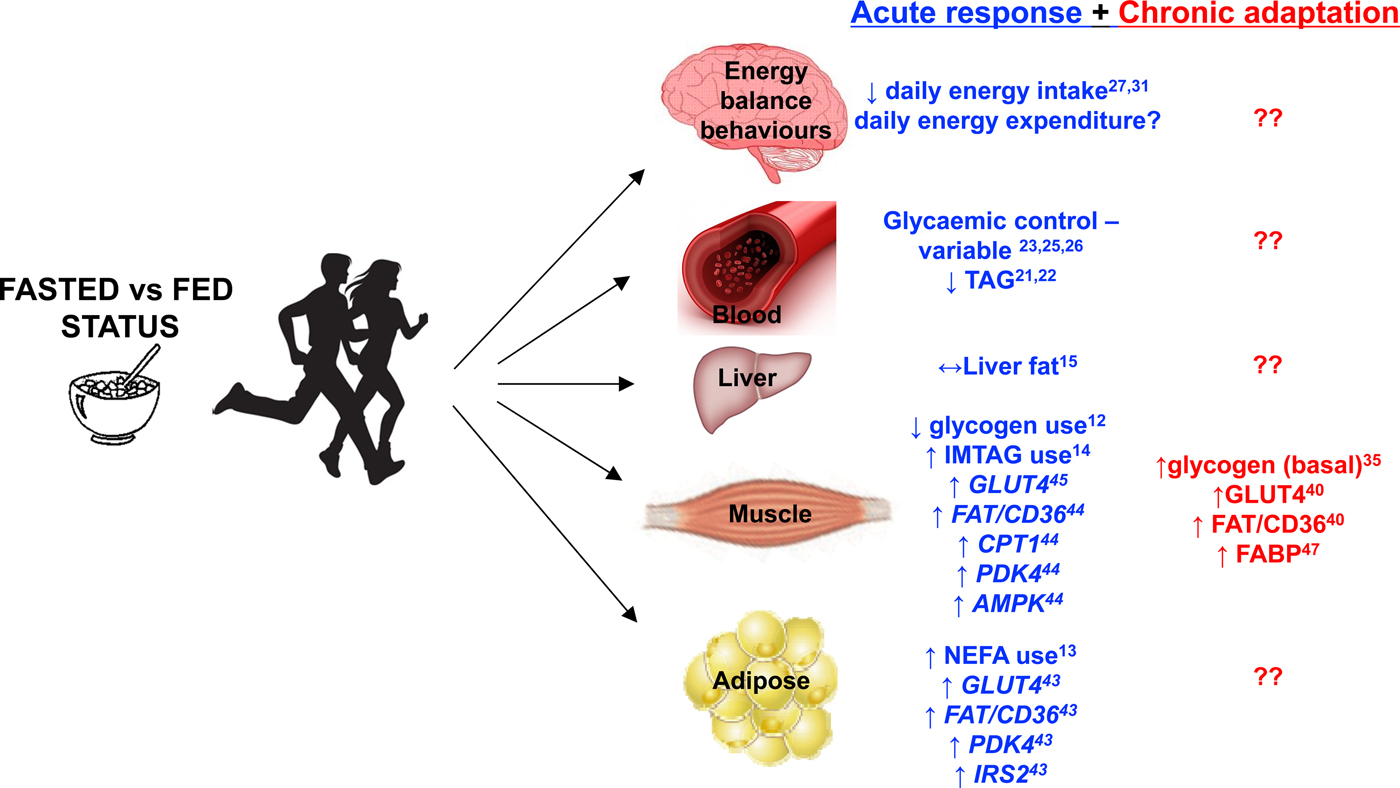
Fig. 1. Major metabolic and behavioural factors influenced by aerobic exercise performed in the overnight-fasted v. fed-state. Acute response refers to a single bout of exercise. Chronic adaptation refers to the culmination of single bouts of exercise over a period of weeks to months as a result of undertaking an exercise training programme. The figure includes results from studies that used a range of study populations and different experimental designs and as such should be regarded as conceptual rather than definitive. Superscript refers to the appropriate supporting reference.
Financial Support
G. A. W. has received research funding from the Engineering and Physical Sciences Research Council (UK), the Biotechnology and Biological Sciences Research Council (UK), GlaxoSmithKline Ltd, Sugar Nutrition UK, Lucozade Ribena Suntory Ltd, Volac International Ltd and the Allen Foundation (USA). J. T. G. has received research funding from The European Society of Clinical Nutrition and Metabolism, The Rank Prize Funds, The Physiological Society (UK), The Biotechnology and Biological Sciences Research Council (UK), The Medical Research Council (UK), Arla Foods Ingredients, Lucozade Ribena Suntory and Kenniscentrum Suiker and Voeding, and has acted as a consultant to PepsiCo. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
The authors had joint responsibility for all aspects of preparation of this paper.



