For the past two decades, there has been considerable interest in the roles of long-chain PUFA in infant growth and development( Reference Swanson, Block and Mousa 1 , Reference Calder 2 ). However, despite an extensive literature describing their contribution to fundamental metabolic functions in brain, immune and cardiovascular systems there has been a lack of consensus amongst the policy-making community on the need for explicit recommendations on dietary intake of DHA and arachidonic acid (ARA) during the early years of life( 3 , 4 ). Most recently the European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies concluded that ARA (20 : 4 n-6) is not necessary in terms of infant formulas when DHA (22 : 6 n-3) is present( 3 ). However, responses to this opinion from infant nutrition scientists and clinicians suggest that there is a lack of evidence to support this view and moreover it may be harmful to provide dietary DHA without adequate levels of ARA( Reference Crawford, Wang and Forsyth 5 ).
A fundamental issue is that most studies have been performed in high-income countries and there is very little research data on the most vulnerable infants living in low-income communities. Moreover, current policy recommendations have tended to be driven by data from randomised controlled trials (RCT) undertaken in institutions located in high-income countries. Policymakers have a responsibility to protect the most vulnerable and this may require balancing the evidence from RCT with observational data obtained from real-world settings. The present paper takes a public health approach to the assessment of the need for ARA and DHA in early life with the aim of providing a pragmatic and coherent way forward that may more effectively serve the needs of the most vulnerable infants and young children.
The benchmark
The WHO recommends that mothers worldwide exclusively breastfeed infants for the child's first 6 months to achieve optimal growth, development and health. Thereafter, they should be given nutritious complementary foods and continue breastfeeding up to age 2 years or beyond( 6 ). It is recognised that additional nutrient-dense complementary foods (liquid and solid) are required following the exclusive breastfeeding period to prevent undernutrition and stunting in the childhood population( 7 ).
Human milk has several compositional components that make it unique with a potent example being the continual presence of significant quantities of DHA and ARA. In contrast, only trace amounts may be found in cow's milk, goat milk, soya milk and rice milk and therefore the level of availability in human milk, as opposed to other animal and plant milks, is highly indicative of their essentiality for human infants. Moreover, there are well-documented evolutionary changes that have led to the development of transport mechanisms for both fatty acids to cross the placenta from the mother to the fetus during pregnancy( Reference Lauritzen and Carlson 8 ) and for the fatty acids to be released from maternal lipid stores and transported to the breast during lactation( Reference Demmelmair, Kuhn and Dokoupil 9 ). Both of these evolutionary mechanisms enhance the delivery of DHA and ARA to the fetus during the prenatal period and to the infant post-birth, a process frequently described as biomagnification( Reference Kuipers, Luxwolda and Janneke Dijck-Brouwer 10 ).
Challenging evolution by proposing that if infants are not being breastfed, they should receive nutritional products that do not contain both DHA and ARA, is a high-risk strategy( Reference Brenna 11 ). With the current benchmark for early nutrition being the composition of breast milk, which contains both ARA and DHA, any discussions that consider a nutritional product that is devoid of these fatty acids need to reflect carefully on the risks and potential harm that may occur as an unintended consequence of the deviation from the evolved nutritional composition of human milk.
Human milk
In 2007, Brenna et al. published data from sixty-five studies, 2474 women and thirty-three countries and reported that the mean concentration of DHA in breast milk (by weight per cent (wt %) of total fatty acids) was 0·32 % (range 0·06–1·4 %) and the mean concentration of ARA was 0·47 % (range 0·24–1·0 %)( Reference Brenna, Varamini and Jensen 12 ). In 2016, Fu et al. provided data on seventy-eight studies from forty-one countries and 4163 breast milk samples and reported that the worldwide mean levels of DHA and ARA in breast milk were 0·37 (sd 0·11) % and 0·55 (sd 0·14) %( Reference Fu, Liu and Zhou 13 ). Both studies showed that the ARA content of breast milk is relatively consistent compared with DHA, the latter being particularly influenced by maternal diet. The levels of DHA were particularly low in populations with the greatest poverty; 0·06 % DHA in Pakistan, Northern Sudan, and 0·10 % DHA in Southern Sudan.
Applying the WHO recommendation on exclusive breastfeeding for 6 months, it is estimated that at 6 months, infants will be receiving approximately 190 mg/d ARA and 130 mg/d DHA, based on a breast milk intake of 850 ml/d and the reported mean concentrations of DHA and ARA in human milk. An infant receiving a formula without supplemented DHA and ARA will clearly have zero consumption from milk feeds during this time.
Complementary foods
Complementary foods tend to have low concentrations of ARA and DHA and their contribution to dietary intakes compared with breast milk is low, and this is evident in both high-( Reference Schwartz, Dube and Alexy 14 ) and low-income countries( Reference Prentice and Paul 15 ). In Germany, dietary intake of ARA and DHA at 9 months was 24 and 28 mg/d respectively at 9 months compared with 72 and 47 mg/d at 6 months when breastfeeding was more prevalent( Reference Schwartz, Dube and Alexy 14 ). Similarly, in Gambia intakes of ARA and DHA at 24 months were both 10 mg/d compared with 90 and 110 mg/d during the period of 0–6 months( Reference Prentice and Paul 15 ). In low-income countries, it is common practice for weaning infants to receive food from the family bowl, which is most commonly of plant origin and has low ARA and DHA content( Reference Prentice and Paul 15 ). In a recent publication, dietary intake of ARA and DHA at the population level, was strongly related to the economic status of the country (Table 1)( Reference Forsyth, Gautier and Salem 16 ). The analysis was based on food consumption data originally collected by the Food and Agriculture Organisation of the United Nations and by utilising food composition tables, the per capita dietary intakes of DHA and ARA were estimated for 175 countries worldwide, with forty-seven classified as developed and 128 as developing. This analysis demonstrated that intakes of both fatty acids varied significantly in relation to the gross national income of the country, with low-income countries having intakes of DHA and ARA which were approximately 20–25 % of that of high-income countries( Reference Forsyth, Gautier and Salem 16 ) (Table 1)
Table 1. Per capita estimated daily intakes of arachidonic acid (ARA) and DHA by gross national income (GNI) of country in 174 countries( Reference Forsyth, Gautier and Salem 16 )
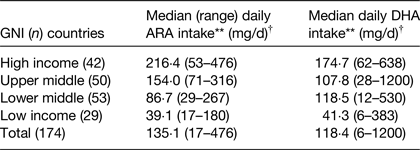
Kruskal–Wallis H test. **P < 0·001.
† The Kruskal–Wallis H test is used to determine whether there are any statistically significant differences in distribution of key outcome variables between three or more variable groups.
Estimates were also made of DHA and ARA intakes during the age period of 6–36 months. This analysis estimated intakes of DHA and ARA from breast milk and complementary foods with median duration of breastfeeding, mean concentration of DHA and ARA in breast milk and mean intakes of breast milk intakes as factors within the calculation( Reference Forsyth, Gautier and Salem 17 ). The data showed that the combined intake of ARA and DHA from both breast milk and complementary foods was lower than previous recommendations( 4 ) and significantly influenced by socio-economic status of the country (Table 2). The data also clearly demonstrated that DHA and ARA intake from complementary foods were exceptionally low in poor-resource countries (Table 3)( Reference Forsyth, Gautier and Salem 17 ).
Table 2. Estimation of arachidonic acid (ARA) and DHA dietary intakes from breast milk (BM) and complementary foods (food) in 6–36 months old children living in seventy-six developing countries ( Reference Forsyth, Gautier and Salem 17 )
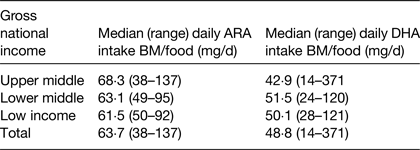
Table 3. Estimation of arachidonic acid (ARA) and DHA dietary intakes from complementary foods in 6–36 months old children living in seventy-six developing countries ( Reference Forsyth, Gautier and Salem 17 )
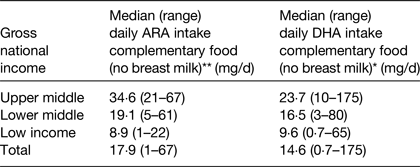
Kruskal–Wallis H test. **P < 0·001, *P < 0·05.
In the low-income countries, the predominant nutritional source of DHA and ARA during the second and third years of life was breast milk, reflecting the limited availability of complementary food sources for these fatty acids. However, beyond the age of 6 months, breast milk does not have the nutritional density to meet the increasing energy and nutrient demands of the growing infant, and failure to provide adequate nutritious complementary foods places the infant at risk of developing undernutrition and stunting( 7 ). The intake of ARA and DHA from complementary foods was particularly low in Nepal, Bangladesh, Ethiopia and Rwanda( Reference Forsyth, Gautier and Salem 16 , Reference Forsyth, Gautier and Salem 17 ).
Birth rate is several-fold higher in low-income countries compared with more affluent populations and this further increases the overall prevalence of long-chain PUFA (LCPUFA) dietary deficiency in these countries. The recent analyses included twenty-five low-income countries, which involved a total population of 644 million, and an average birth rate of thirty-four births per 1000 population. It is estimated that each year in this cohort of countries, approximately twenty-two million infants are born and they are at significant risk of LCPUFA insufficiency ( Reference Forsyth, Gautier and Salem 16 , Reference Forsyth, Gautier and Salem 17 ).
Importance of a continuum of DHA and arachidonic acid intake during early life
In 1992, seminal work by Manuela Martinez demonstrated that during the latter part of pregnancy and during the first 2 years of life there is a rapid accumulation of both DHA and ARA in the developing brain( Reference Martinez 18 ). Shortly after this publication there were two papers showing that the DHA content of the infant brain was higher in breastfed infants compared with infants who had received infant formulas that were devoid of both DHA and ARA( Reference Makrides, Neumann Byard and Simmer 19 , Reference Farquharson, Jamieson and Abbasi 20 ). It was subsequently shown in an RCT that infants receiving a formula with added DHA and ARA had higher blood levels of DHA and ARA during the first year of life compared with infants who received a formula that was not supplemented with these fatty acids( Reference Birch, Castañeda and Wheaton 21 ). These data clearly highlight that dietary intake of DHA and ARA significantly influence fatty acid status at this critical period of development and a continuum of DHA and ARA intake during early life should be a key nutritional objective.
It is also evident that after 6 months of age the balance between breast milk intake and complementary feeding, is critical. Although breast milk is an important source of DHA and ARA the volume of breast milk intake will decline to allow capacity for high-density complementary foods, and unless these foods contain adequate levels of ARA and DHA there will be a depletion in these fatty acid levels( Reference Birch, Castañeda and Wheaton 21 ). It is therefore important that not only should complementary foods in developed and developing countries be commenced around the age of 6 months, but also the continuum of DHA and ARA intake needs to be maintained. This can be achieved by ensuring that complementary foods (both liquid and solid), contain these fatty acids.
Inter-dependency between DHA and arachidonic acid
There is a biochemical interdependency between DHA and ARA as they share the same metabolic pathway that enables desaturation and elongation of precursor fatty acids. Studies have shown that the endogenous synthesis of both DHA and ARA in early life is insufficient to meet metabolic requirements and that blood and tissue concentrations decrease rapidly after birth if exogenous supplies are not adequate( Reference Brenna, Salem and Sinclair 22 – Reference Carnielli, Simonato and Verlato 24 ). It is also important to note the data from animal and human studies that have shown that alteration of the intake of either DHA or ARA can significantly influence the endogenous synthesis of the other. For example, when pregnant rats were fed marine oil with high EPA/DHA and a limited supply of ARA, ARA levels in brain phospholipids in the offspring were severely decreased by nearly 50 % compared with controls( Reference Yonekubo, Honda and Okano 25 ). Similar effects, a 25 % decrease in ARA blood levels, have been noted in human infants when formulas were supplemented with DHA but not ARA. The balance between DHA and ARA was significantly different from that of breast milk( Reference Makrides, Gibson and Udell 26 ). This evidence indicates that an imbalance of the DHA and ARA content of infant formula should not be recommended without clear evidence of its short- and long-term safety.
Scientific evidence
There is a wealth of evidence from experimental and animal studies that DHA and ARA have critical functions in human metabolism. It is evident that DHA has a key role in visual and cognitive development( Reference Koletzko, Boey and Campoy 27 ) and that ARA is a key intermediary in the synthesis of leukotrienes, prostacyclines, thromboxanes and PG that have important roles in brain, immune and cardiovascular function( Reference Hadley, Ryan and Forsyth 28 ). Translating this scientific evidence into clinical benefit as evidenced by RCT has been challenging; however, current evidence indicates that cognitive functions in the fields of attention control, information processing and problem solving are enhanced in intervention groups( Reference Koletzko, Carlson and van Goudoever 29 ). A major concern is that the RCT have almost entirely been undertaken in high-income countries, with most publications being from institutions in North America, Europe and Australia. Undertaking studies in populations that are reasonably endowed with DHA and ARA will undoubtedly impact on the outcomes of the study and this is reflected in the conclusions of systematic reviews. The relevance of the current clinical studies and their related systematic reviews needs to be considered in a context of a marked global variation in dietary intakes of DHA and ARA with the lowest dietary intakes being in the poorest countries( Reference Forsyth, Gautier and Salem 16 , Reference Forsyth, Gautier and Salem 17 ).
Clinical intervention studies tend to take place in the context of a relatively, well-defined biological environment with a narrow variation in individual responses, whereas public health interventions take cognisance of the wide spectrum of social difference in the study population. Long-term outcomes are particularly important in studies that are attempting to relate nutrition in early life to health in later childhood and adulthood; however, in longitudinal RCT, data on outcome measures are frequently subject to attrition rates that undermine the integrity of the randomisation model. While RCT, notably cluster RCT, can be designed to evaluate some complex public health programmes, often they are not feasible because of the practical or resource constraints and consequently well-conducted RCT are relatively rare in public health.
Public health perspective
Public health focuses on promoting or protecting health in communities and national populations, and an overarching priority is to reduce health inequalities. The public health considerations of a specific health issue within a population will generally include the perceived magnitude and importance of the problem, the potential effectiveness and harms of an intervention, the feasibility of its implementation, and its political and public acceptability( Reference Rychetnik, Frommer and Hawe 30 ).
If these factors are considered in a public health approach to DHA and ARA intakes within a population, it is likely to be concluded that the evidence indicates that the perceived issue is of considerable magnitude, involving many countries and with many millions of infants and young children receiving low dietary intakes of DHA and ARA. The intervention of DHA and ARA supplementation of infant and follow-on formulas has already been tried and tested in many millions of infants over the past two decades and no regulatory body has reported any concerns regarding potential harm; the implementation is feasible as there are already formulas and complementary foods that are available and widely distributed in the public domain, especially in high-income countries. Finally there is increasing awareness within the general public of the potential health benefits of LCPUFA, especially DHA, at different stages of the life cycle. In addition, most governments are committed to reducing health inequalities and the available evidence clearly indicates that dietary intakes of DHA and ARA are significantly influenced by socio-economic factors within the population.
Although infants and their families living in medium to low-income countries are at greatest risk of dietary deficiencies of ARA and DHA in early life, shorter duration of breastfeeding and the early introduction of low content of ARA and DHA in complementary foods can decrease dietary intake of ARA and DHA in infants living in more affluent communities. It is therefore evident that global policy recommendations need to be inclusive of all countries.
Conclusions
Relating early life nutritional interventions to later life health outcomes is always going to be challenging and all research methods will have their limitations. There is a real concern that null RCT of LCPUFA supplementation may be misinterpreted to indicate that low dietary intake of DHA and ARA will be of no consequence to even the most vulnerable infants. Recent evidence indicates that a high proportion of the global childhood population may be at risk of LCPUFA deficiency in early life( Reference Forsyth, Gautier and Salem 16 , Reference Forsyth, Gautier and Salem 17 ).
It is important that the body of research available to policymakers reflects the diversity of the populations being considered and that studies need to be inclusive and focus on those individuals and communities that are at greatest risk. In some settings, observational studies may represent the most feasible and appropriate study designs for identifying ‘at risk’ populations and evaluating the public health interventions.
The principal objective of recommendations on dietary DHA and ARA in early life should be to provide a safety net for the most vulnerable infants worldwide. In relation to infant formulas and follow-on formulas, the availability of DHA and ARA should be mandatory to protect infants who do not receive adequate supplies of breast milk and to prevent further nutritional inequality between high- and low-income countries. Levels of DHA and ARA in infant formulas and follow-on formulas should equate to median levels of DHA and ARA in breast milk and a minimum level needs to be determined. For countries that can clearly evidence that infants and young children consume adequate intakes of DHA and ARA during early life, an optional recommendation for these countries may be considered.
Financial Support
None.
Conflicts of Interest
S. F. undertakes consultancy work for DSM Nutritional Products. S. G. and N. S. are employees of DSM Nutritional Products and the company produces ARA and DHA.
Authorship
All authors were involved in the planning and writing of the paper.






