The human gut microbiota
The human gut microbiota is a diverse collection of microbes inhabiting the gastrointestinal tract, encompassing up to 1000 different species and with a gradient of concentration going from about 102–3 bacteria/gram of content in the stomach to 105 bacteria/gram in the duodenum and jejunum, 108 bacteria/gram in the ileum and 1011–12 bacteria/gram in the large intestine.
DNA-based tools for measuring the composition and function of gut microbiota (i.e. next-generation sequencing, quantitative polymerase chain reaction, fluorescent in situ hybridisation) have largely allowed us to overcome the technical limitations of direct cultivation of microbes and provided large amounts of information on gut microbial profiles and activities(Reference Lepage, Leclerc and Joossens1). The main resident bacterial populations in the human gut belong to the phyla Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria and Verrucomicrobia. Gut microbiota composition has also been described in terms of a limited number of ‘enterotypes’, according to which the human gastrointestinal tract is colonised by loose groups of co-occuring microbes centred around the genera Prevotella, Bacteroides and/or Ruminococcus (Reference Arumugam, Raes and Pelletier2). Despite the relatively few bacterial phyla, the gut microbiota is a highly complex ecosystem, with each bacterial species and even strain being capable of carrying out specific functions within the diverse microbial community. Resident bacteria colonise the human body in a highly host-specific manner, meaning that there is elevated inter-individual variation in terms of species and strain composition, abundance and metabolic output(Reference Arumugam, Raes and Pelletier2). This specificity is also determined by host genotype, age, sex and health state(Reference Bolnick, Snowberg and Hirsch3). Many disease states are characterised by alterations in microbiota composition and metabolic function(Reference Ottman, Smidt and de Vos4). Nevertheless, gut microbes are deeply affected by the host's lifestyle. The environmental input represented by physical activity(Reference O'Sullivan, Cronin and Clarke5), diet(Reference Rowland, Gibson and Heinken6) and circadian rhythm(Reference Marcinkevicius and Shirasu-Hiza7, Reference Deaver, Eum and Toborek8) has been shown to drive changes in microbial profiles and activities, such as production of fermentation end-products such as SCFA, biotransformation of dietary compounds (i.e. plant phenolics) and xenobiotics, conversion of bile acids (BA) and modulation of circulating BA pool(Reference Long, Gahan and Joyce9) (Fig. 1).
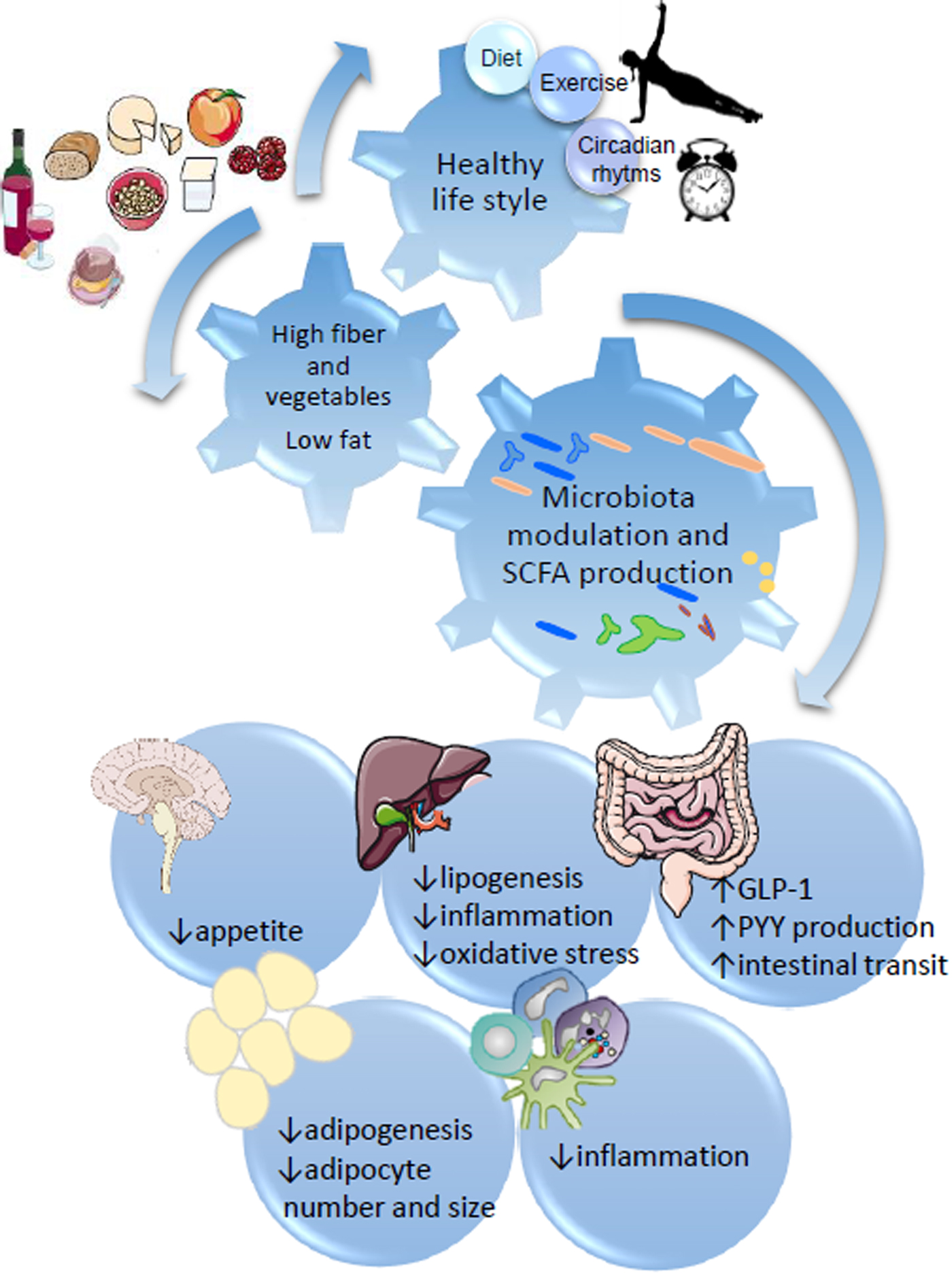
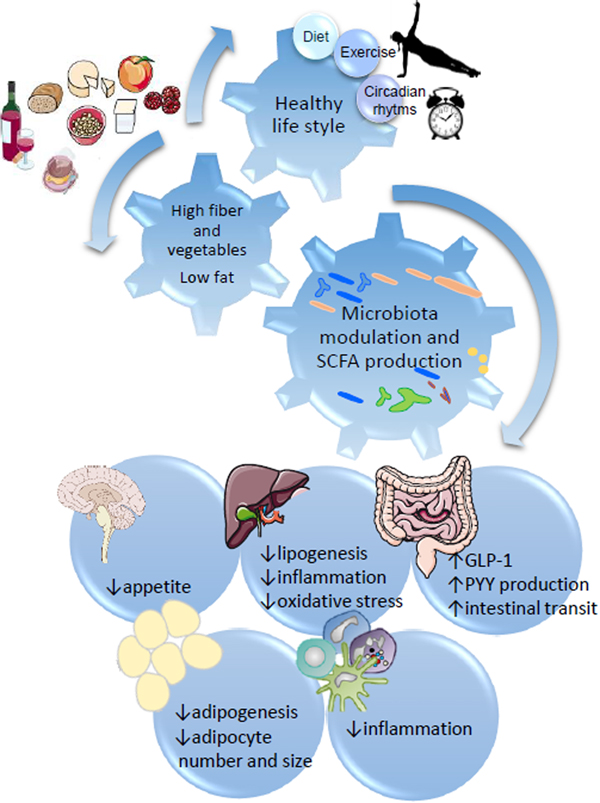
Fig. 1. SCFA regulation of metabolic health through diet:gut microbiota interaction. Healthy habits, including diet (i.e. Diet high in fibre and plant bioactives), physical exercise and appropriate sleep/wake alternation directly influence microbial fermentation and other microbial metabolic activities. Production of SCFA, biotransformation of plant phenolics, conversion of bile acids and metabolism of xenobiotics directly influence systemic metabolism, including central appetite regulation (i.e. through secretion of intestinal hormones GLP-1 and PYY), lipogenesis and adipogenesis, and modulate systemic and hepatic inflammation.
The gut microbiota contributes to human physiology mainly through immune and metabolic functions. Resident bacteria constitute an effective barrier from pathogen invasion through direct competition for nutritional substrate and ecological niches as well as the production of antimicrobial substances. Gastrointestinal microbes contribute to immune system maturation, development of immune tolerance mechanism and maintenance of epithelial barrier integrity(Reference Rizzetto, Fava and Tuohy10). Gut bacteria provide the human organism with additional metabolic enzymes, which allow fermentation and biotransformation of dietary compounds, which would otherwise remain undigested and generate metabolites that exert their physiological role systemically(Reference Marchesi, Adams and Fava11, Reference Flint, Scott and Louis12). Saccharolytic fermentation takes place in the proximal part of the colon, while proteolytic fermentation appears to be located in the distal region of the large intestine. The result of carbohydrate fermentation is the production of beneficial SCFA, while, conversely, proteolytic fermentation generates amines, ammonia, N-nitroso compounds, sulphides, indoles and other toxic or potentially carcinogenic compounds(Reference Rowland, Gibson and Heinken6).
The main SCFA produced in the human colon are acetate, propionate and butyrate, in a molar ratio about 3:2:2. Acetate is the main SCFA produced by gut microbes and is an essential nutrient for the growth of many bacteria. Once it is produced acetate is absorbed and enters systemic circulation to the peripheral tissues, where it serves as a substrate for cholesterol metabolism and lipogenesis, as well as satiety regulation. Recently, acetate has been shown to play an important role in the browning of white adipose tissue, thermogenesis and protection from obesity(Reference Weitkunat, Stuhlmann and Postel13, Reference Jocken, González Hernández and Hoebers14). Propionate is absorbed through the portal vein to the liver and is converted into glucose through hepatic gluconeogenesis. It also plays an important role in regulating the inflammatory and endocrine output of adipose tissue, again protecting from obesity and metabolic disease(Reference Al-Lahham, Roelofsen and Priebe15). Butyrate is the main source of energy for enterocytes and it has an anti-cancer activity through the promotion of apoptosis and repression of proliferation by inhibition of histone de-acetylation. Butyrate is also involved in maintaining the integrity of the gut wall, protecting against ‘leaky gut’ and associated inflammation and regulation of T-cell function and inflammatory response(Reference Kim, Park and Kim16).
Biotransformation of dietary plant polyphenols by the gut microbiota through hydrolysis, dehydroxilation, demethylation and other enzymatic activities generates polyphenols catabolites with increased bioavailability, which are then absorbed through the intestinal mucosa and carry out biological effects in several districts of the organism(Reference Ozdal, Sela and Xiao17). Small phenolic compounds originated from polyphenol metabolism by gut bacteria were shown to have an anti-inflammatory, detoxifying, anti-cancer and anti-protein glycation activity in vitro and in laboratory animals(Reference Del Rio, Rodriguez-Mateos and Spencer18).
The gut microbiota explicates its metabolic function also through conversion of primary BA to secondary and tertiary BA, as a result of enzymatic activities such as bile salt hydrolase and 7-α-dehydroxilase(Reference Ridlon, Harris and Bhowmik19). Circulating BA mediate their systemic effect through stimulation of BA receptors (i.e. nuclear receptors, such as farnesoid X receptor (FXR), vitamin D receptor, pregnane X receptor and G-protein coupled membrane receptors, such as the G protein-coupled BA receptor GP-BAR1/TGR5, muscarinic receptor, Formyl-peptide receptors receptor) in several tissues of the body, including smooth and striated muscle, heart, macrophages, neurons and adipose tissue. BA stimulated cell signalling involves not only the entherohepatic axis (i.e. regulation of BA synthesis and turnover) but also lipid metabolism, energy expenditure and glucose homeostasis. Different BA were previously shown to differentially impact on the earlier mentioned physiological effects(Reference Joyce and Gahan20). The pool of circulating BA strongly depends on gut microbiota activity and dietary input can apparently influence both hepatic BA neosynthesis and intestinal production of secondary and tertiary BA(Reference O'Keefe, Li and Lahti21).
Dietary compounds deeply affect the growth and metabolism of gut bacteria, since fermentation of nutrients is one core function of the human gut microbiota. The study of the contribution of microbes to systemic metabolic pathways has been recently made possible thanks to the employment of integrating ‘-omics’ technologies, such as metabolomics, in addition to microbiota metagenomics analysis(Reference Nicholson, Holmes and Kinross22). Particularly through measuring the gut microbiota response to a definite dietary component or food in terms of generated microbially-derived metabolites, as well as concomitant changes in bacterial composition and systemic clinical parameters, it is now possible to gain information regarding bacterial functions in the human organism.
Gut microbiota and obesity
The concept of microbial dysbiosis, as firstly introduced to explain the shifts in gastrointestinal bacterial makeup and in relation to intestinal dysfunction characteristic of inflammatory bowel disease, has more recently been extended to describe the differences in gut microbiota observed between healthy individuals and individuals affected by different chronic extra-intestinal diseases, including CVD, obesity, metabolic syndrome, autoimmune pathologies (i.e. psoriasis, rheumatoid arthritis, asthma), allergies, neurodegenerative disease and some cancers(Reference Carding, Verbeke and Vipond23). Research done on the gut microbiota over the last decade made progress in understanding the role of resident bacteria in relation to Western metabolic diseases linked to overweight and associated pathologies. Studies comparing gut microbial profiles of obese and lean mice described an ‘obese microbiota phenotype’, which was shown to differ from the ‘lean microbiota phenotype’ for reduced prevalence of Bacteroides, one of the main bacterial phylum in the mouse and in the human gut and for concomitant increased prevalence of the phylum Firmicutes(Reference Ley, Turnbaugh and Klein24–Reference Turnbaugh, Ley and Mahowald26). Further analysis identified specific bacterial candidates that differentiate obese and lean individuals at a higher taxonomic level within the Firmicutes phylum. The Mollicutes class was found higher in Western diet-induced obese mice compared with controls, and its abundance was reduced especially after dietary intervention with carbohydrate restriction and weight loss(Reference Turnbaugh, Bäckhed and Fulton27). Authors suggested that the observed microbiota profile in mice consuming a Western-style diet was due to the functional advantage of Mollicutes to process high-sugar Western foods(Reference Turnbaugh, Bäckhed and Fulton27). These studies also reveal the contribution of diet in shaping gut microbiota in relation to obesity and obesity-correlated diseases. The initial animal studies showing altered Firmicutes:Bacteroidetes ratio in genetically obese leptin deficient ob/ob mice were confirmed in obese human subjects and raised attention towards gut microbiota monitoring in association with metabolic health and obesity. Also, the role of diet in inducing obesity-associated gut microbiota changes was highlighted by showing how the ‘obese microbiota phenotype’ reverted to ‘lean microbiota phenotype’ upon dietary restriction (i.e. carbohydrate- or fat- restricted diet). Work by Cani and Delzenne focused on the importance of low-grade chronic inflammation linked to obesity. Cani et al.(Reference Cani, Amar and Iglesias28) showed that in high-fat-fed obese mice had circulating levels of bacterial lipopolysaccharide, defined as ‘metabolic endotoxemia’, as well as metabolic syndrome symptoms (i.e. glucose homeostasis impairment, insulin resistance, inflammation) and dysbiotic microbiota (particularly decreased Bifidobacterium spp). The authors hypothesised increased intestinal permeability and consequent ‘leaky gut’ as the cause of metabolic endotoxemia. In further studies, Cani and coworkers demonstrated that restoration of gut barrier integrity and lowering of circulating lipopolysaccharide concentration could be achieved by dietary supplementation of dietary fibre (i.e. oligofructose) to a high-fat diet(Reference Cani, Neyrinck and Fava29). High-fat + oligofructose restored the levels of Bifidobacterium spp and promoted the growth of Akkermansia muciniphila, increased faecal SCFA, promoted epithelial barrier integrity through tight junction proteins expression, decreased plasma lipopolysaccharide, increased satiety through gut hormones expression (i.e. glucagon-like peptide-1, glucagon-like peptide-2, Peptide YY (PYY)), stimulated endocannabinoid system, and improved glucose homeostasis(Reference Cani, Neyrinck and Fava29–Reference Muccioli, Naslain and Bäckhed32). Several human studies confirmed the increased intestinal permeability in association with obesity(Reference Amar, Burcelin and Ruidavets33, Reference Rainone, Schneider and Saulle34).
Obesity-associated dysbiosis was also seen in correlation with the altered bacterial metabolic activity of gut bacteria, represented by changes in the production of fermentation end-products SCFA. The main SCFA, acetate, propionate and butyrate, were seen to be raised in faecal samples of obese animals and human subjects(Reference Turnbaugh, Ley and Mahowald26, Reference Fava, Gitau and Griffin35), although the same trend was not observed in plasma. In obese mice, with spontaneous metabolic syndrome, the concentration of plasma SCFA was observed to be significantly lower compared with the lean counterparts(Reference Nishitsuji, Xiao and Nagatomo36). Higher plasma SCFA were seen as positively affecting gut hormone secretion and satiety regulation. Inulin consumption was shown to promote SCFA production and fat oxidation in overweight men(Reference van der Beek, Canfora and Kip37). Raised plasma acetate, propionate and butyrate concentration, following intraperitoneal infusion of SCFA in similar concentrations to the ones reached after inulin consumption, was also shown to improve fat oxidation, increase energy expenditure, induce gut satiety hormone PYY and induce lipolysis(Reference Canfora, van der Beek and Jocken38). These studies highlight the importance of fermentation to mediate diet:gut microbiota interaction in obesity and associated conditions. They also provide new mechanistic insight to the now recognised inverse relationship between dietary fibre intake and obesity/metabolic disease and give further confirmation on the beneficial role of foods which stimulate SCFA production. However, they also raise questions about the validity of faecal concentration of SCFA as a biomarker of intestinal fermentation, as discussed by Verbeke et al.(Reference Verbeke, Boobis and Chiodini39). SCFA concentration measurement in faecal samples is not representative of SCFA colonic production and absorption. Therefore, considering the effect of intestinal fermentation end-products on systemic metabolism, appetite regulation, lipid and glucose homeostasis, immunity and inflammation, measurement of SCFA production should be carried out by integrating information coming from metabolite analysis in different compartments of the organisms, in order to gain knowledge on the nutrikinetic of foods involving gut microbial metabolism.
Gut microbiota: healthy diet interaction and metabolic health
Healthy dietary habits, specifically diets with low energy coming from fat and protein and high energy coming from carbohydrates, specifically complex carbohydrates and dietary fibre, are widely recognised for their protective effect against CVD risk and there is mounting evidence that many of the effects of these diets include a contribution from gut microbial metabolic activities. Epidemiological and dietary interventions have extensively demonstrated that a Mediterranean-style diet lowers CVD, improves mental health and reduces inflammation and cancer(Reference Bonaccio, Di Castelnuovo and De Curtis40–Reference Schwingshackl and Hoffmann43). Mediterranean dietary patterns are characterised by low energy intake, energy intake largely derived from carbohydrates and less from fats and proteins, the prevalence of complex carbohydrates over simple carbohydrates/sugars and of monounsaturated and polyunsaturated fats over saturated fats, as well as fish, pulses and dairy over red meat. Dietary fibre and polyphenols are both pillars of the Mediterranean diet and have both been shown to independently contribute to lower disease risk and mortality(Reference Bonaccio, Di Castelnuovo and Costanzo44, Reference Pounis, Costanzo and Bonaccio45). Such dietary traits are not unique to the Mediterranean diet but are also found in some traditional oriental diets, such as the Okinawan diet in Japan, characterised by life-long energy restriction, low meat intake, high vegetable-based foods and high fish consumption. Adherence to Okinawan diet was observed to be protective against all-cause mortality and age-related diseases(Reference Willcox, Willcox and Todoriki46). This is logical from a molecular and biochemical point of view, as these diverse diets share common characteristics now known to regulate body weight and metabolic disease risk, such as low energy intake throughout life and high intake of dietary fibre, polyphenols and other plant bioactives.
When trying to find a link between healthy high-fruit and vegetables diets and gut microbiota modulation of systemic health, we need to consider that whole vegetable foods are made up of a complex mixture of bioactive compounds such as dietary fibres, plant sterols and polyphenols, as well as vitamins and minerals, which render the response of each individual and individual's gut microbiota to each food highly specific. The effects of plant-derived foods on the gut microbiota are multiple and intertwined. Beneficial effects are likely to be mediated by more than one plant bioactive compounds, maybe even acting synergistically, and by microbial consortia, rather than individual species and strains. Epidemiological and intervention studies succeeded to consistently prove the connection between high fruit and vegetables consumption, beneficial gut microbial profiles and health(Reference Tuohy, Conterno and Gasperotti47).
Recent dietary interventions in subjects at high risk of developing CVD have shown that diets high in fruit and vegetables improve cardiovascular health and impact on the gut microbiota(Reference Guasch-Ferré, Hu and Ruiz-Canela48, Reference Klinder, Shen and Heppel49). In particular the FLAVURS study showed that increasing the consumption of fruit and vegetables up to six 80 g portions daily caused a significant increase in total urinary flavonoids, vitamin C and other phytochemicals, as well a significant increase in total dietary fibre intake, calculated as NSP food diary estimate(Reference Chong, George and Alimbetov50, Reference Macready, George and Chong51). Gut microbial C. leptum-R. bromii/flavefaciens, Bifidobacterium and Bacteroides/Prevotella all increased in people consuming high fruit and vegetables(Reference Klinder, Shen and Heppel49).
A healthy diet, specifically diet with a low energy coming from fat and protein and a high energy coming from carbohydrates, specifically complex carbohydrates and dietary fibre, was previously associated with a specific gut microbiota composition. De Filippo et al.(Reference Filippo, Cavalieri and Paola52) showed that Burkina Faso children, who consumed a traditional high-fibre, high-vegetables/pulses/tuberous and low-protein, low-fat diet, harboured a gut microbiota with significantly higher Bifidobacterium and Prevotella spp, and significantly lower prevalence of potentially pathogenic Enterobacteriaceae (Klebsiella, Salmonella, Shigella and Escherichia coli spp) compared with Italian children consuming a high-fat high-simple sugar diet(Reference Filippo, Cavalieri and Paola52). Interestingly, Burkina Faso individuals were also colonised by the cellulose-degrader Xylanibacter, thus reflecting the role of diet in favouring colonisation of the human gastrointestinal tract by bacteria with a specific metabolically advantageous function. High prevalence of Prevotella spp was described in a further study as one of the main microbial features that differentiated individuals following a long-term low-fat/high-carbohydrates diet from individuals consuming a high-fat/high-protein/low-fibre diet(Reference Wu, Chen and Hoffmann53). Differences in gut microbiota composition were observed in another study that compared geographical and cultural setups in South America and in the USA. The study showed that the differences could be explained by the populations’ dietary habits. Particularly high intake of carbohydrate-based traditional foods was thought to be responsible for the distinction of Amerindians and Malawians from US Americans in terms of microbiota profile, consisting in higher abundance of Prevotella spp, as well as Ruminococcus spp, Lactobacillus spp and some Actinobacteria in non-US Americans compared with US Americans(Reference Yatsunenko, Rey and Manary54). One long-term human dietary intervention study on the effect of different types of fat and carbohydrates on the gut microbiota showed that high-carbohydrate/low-fat diets increased faecal Bifidobacterium and Bacteroides spp compared with high-fat/low-carbohydrate diet(Reference Fava, Gitau and Griffin35).
In summary diet low in complex carbohydrates and plant-derived compounds, and high in simple sugars, fat and proteins modulate the gut microbiota by promoting the growth of detrimental facultative anaerobic bacteria, such as members of the Enterobacteriaceae family, and of potentially opportunistic pathogens, such as Bacteroides spp. Conversely diets high in complex carbohydrates and fibre favours the growth of polysaccharide degraders and related bacteria, such as Prevotella, Bifidobacterium, Lactobacillus Ruminococcus spp. and some Lachnospiraceae.
Dietary fibre
Many, although not all, dietary sources of fibres are ‘prebiotic’, since they selectively stimulate the growth and/or the activity of some beneficial commensal bacteria in the gut(Reference Gibson, Hutkins and Sanders55). Plants represent the highest font of dietary fibres, due to their structural complex polysaccharides, some of which are insoluble (i.e.: cellulose, hemicellulose, resistant starch, xylans, lignin) and some soluble (pectins, β-glucans, gums, mucilage, fructans). Vegetable-derived fibres are resistant to human enzyme digestion and often represent a substrate for bacterial fermentation in the large intestine. The result of dietary fibre fermentation is a cascade of events with the gut microbiota as a protagonist, including increase of intestinal biomass (particularly due to promoted growth of some beneficial bacterial populations, such as bifidobacteria and lactobacilli), production of SCFA, lowering of intestinal pH, reinforcement of tight junction and intestinal epithelial integrity, anti-proliferative effect on colonocytes, binding of intestinal carcinogens, binding and biotransformation of polyphenolic compounds(Reference Macfarlane and Macfarlane56). The effect of prebiotics on the human gut microbiota is an increase in the levels of beneficial bacteria belonging to the Bifidobacterium and Lactobacillus genera, but also of other commensal bacteria that possess carbohydrate degrading enzymatic milieu, such as members of the Bacteroidetes phylum(Reference Flint, Scott and Duncan57). Considering the complexity of gut microbiota organisation, prebiotic dietary fibres exert their effect at multiple levels and on the gut microbiota as a whole, not just targeting individual bacterial genera, which are commonly associated with the final benefits coming from prebiotics. Mechanisms of cross-feeding are involved in the prebiotic fermentation of many dietary fibres and in most cases, the starting nutritional substrate for one group of bacteria is the metabolic end-product of other bacterial populations(Reference Rios-Covian, Gueimonde and Duncan58). The diversity of fibres coming from different sources drives the diversity of response of gut microbes and, consequently, diversity of microbial metabolic input to human metabolism. Generalisation of the concept of prebiotic effect ignores the specificity of action of different prebiotic-like dietary fibres and underestimates the importance of dietary diversity to make the most of gut microbiota metabolic potential. Different mechanisms of action of distinct prebiotics were recently shown by Weitkunat et al.(Reference Weitkunat, Stuhlmann and Postel13), through comparison of the effect of supplementation with prebiotic inulin or guar gum to high-fat fed mice. The authors showed that different bifidobacterial populations were stimulated by two different prebiotics, with inulin promoting B. animalis and guar gum increasing B. pseudocatenulatum, respectively. Also, different impact on obesity-related biomarkers was observed between the two prebiotics, since inulin, but not guar gum, induced beige markers (Pgc1α, Ucp1, Cidea) in white adipose tissue, thus promoting thermogenesis, reduced hepatic steatosis, decreased high-fat diet-induced weight gain and improved insulin sensitivity. In the same study, a similar effect was observed when mice were supplemented with the SCFA acetate and propionate, thus suggesting the role of bacterial fermentation end-products of bifidobacteria (i.e. acetate) to mediate the beneficial effects of inulin(Reference Weitkunat, Stuhlmann and Postel13).
Different outcomes on gut microbiota composition, microbial metabolic response and systemic consequences were previously observed in human dietary supplementation studies by employing different dietary sources of fibres. Whole grain foods, defined as foods containing the endosperm, the germ and the bran components of the grain in the same relative proportion as in the intact kernel(Reference van der Kamp, Poutanen and Seal59), constitute a font of fibre, especially arabinoxylans, β-glucans and resistant starch, as well as bioactive compounds, mainly polyphenols. Significant increases of faecal bifidobacteria and in some cases of lactobacilli species were observed after dietary intervention with whole grain wheat(Reference Costabile, Klinder and Fava60, Reference Christensen, Licht and Kristensen61), whole grain oats(Reference Connolly, Tzounis and Tuohy62, Reference Connolly, Lovegrove and Tuohy63) and whole grain maize(Reference Carvalho-Wells, Helmolz and Nodet64). Supplementation with oats to the diet, as shown by Connolly et al.(Reference Connolly, Tzounis and Tuohy62), induced a decrease in total- and LDL-cholesterol levels concomitantly with the prebiotic effect. Dietary intervention with a diverse combination of whole grains has also shown to increase other beneficial bacterial groups, besides bifidobacteria and lactobacilli, such as high butyrate-producers within the C. leptum cluster(Reference Ross, Bruce and Blondel-Lubrano65). Only rarely quantitative changes in faecal bacterial composition after whole grain consumption was accompanied by an increase in faecal SCFA. Barley supplementation to the diet was shown capable to induce a significant increase in faecal SCFA, together with augmented Clostridiaceae, Ruminococcus spp and Roseburia sp(Reference Angelis, Montemurno and Vannini66). These observation highlight the importance of monitoring the fate of SCFA produced by intestinal bacterial saccharolytic fermentation, since sometimes the excreted SCFA are not informative of the concentration of circulating plasma SCFA and of the impact that SCFA have systemically. Also, the dose of fibre, as well as the origin, is an important factor to be considered when trying to understand the effect that diverse sources of dietary fibre have on the gut microbiota in relation to metabolic health. Zhang et al.(Reference Zhang, Yin and Li67) demonstrated how a very high intake of dietary fibre through whole grain foods, legumes, prebiotic oligosaccharides as well as different types of fruit and vegetables, in order to deliver an estimate dose of 50 grams of daily intake of fibre, was efficacious to induce deep changes in gut microbiota composition (increased prevalence of fibre degraders, including Bifidobacterium genus), together with marked improvement of obesity-associated characteristics (weight loss and improvement of dyslipidaemia) after 3 months of dietary intervention in genetically obese children(Reference Zhang, Yin and Li67).
Polyphenols
Polyphenols are plant secondary metabolites that can be found in a variety of vegetable foods, including fruit, vegetables, beverages (coffee, tea and wine), cocoa, herbs, cereals, seeds. Once ingested their bioavailability strongly depends on the physical structure of the plant that carries them and on biotransformation by the gut microbiota. After ingestion nearly the totality of plant phenolics reach the large intestine unabsorbed, where they accumulate to reach a millimolar concentration range(Reference Tuohy, Conterno and Gasperotti47). Thanks to colonic microbes polyphenols are turned into bioactive compounds which explicate their effect systemically. However, similar to dietary fibres, polyphenols are a very large and heterogeneous group of compounds, which require an equally heterogeneous microbial enzyme setting for their cleavage, hydrolysis and dihydroxylation in order to become bioactive, as found in their circulating form. Polyphenols also modulate the composition of gut microbiota in a prebiotic-like manner, by stimulating the growth of beneficial bacteria and by inhibiting pathogens(Reference Ozdal, Sela and Xiao17). Human studies on the effect of polyphenols on gut microbiota have suggested that different type of polyphenols with different origin specifically stimulate distinct gut microbial populations. However, a consistent stimulatory effect on the growth of Bifidobacterium and Lactobacillus spp was observed after intake of isoflavones, proanthocyanidins, ellagitannins, stilbenes (i.e. resveratrol) at a concentration between 100 and 1000 mg daily(Reference Jiménez-Girón, Queipo-Ortuño and Boto-Ordóñez68). An increase in Ruminococcaceae, Akkermansia and Clostriudium coccoides spp, was observed respectively in association with consumption of proanthocyanidins, pomegranate ellagitannins and isoflavones(Reference Duda-Chodak, Tarko and Satora69, Reference Sauceda, Pacheco-Ordaz and Ayala-Zavala70). Health effects of high polyphenol food supplementation were shown in a dietary supplementation study with a cocoa drink, where beneficial modulation of gut microbiota (i.e. increased bifidobacteria and lactobacilli and decreased Clostridium hystolyticum spp) was observed alongside a significant reduction of circulating TAG and C-reactive protein in healthy human subjects(Reference Tzounis, Rodriguez-Mateos and Vulevic71). Similarly, red wine polyphenols induced an increase in beneficial bacterial populations (Bifidobacterium, Lactobacillus, Bacteroides, Clostridium coccoides, Eubacterium rectale, Eggertella, Prevotella) and reduction of plasma TAG and cholesterol, as well as decreased systolic blood pressure(Reference Queipo-Ortuño, Boto-Ordóñez and Murri72).
One limitation of studies of the impact of polyphenol consumption on the gut microbiota in relation to human health is that they often fail to monitor the fate of the bioactive form of polyphenols, such as small phenolic compounds and other microbial metabolites, generated by bacterial transformation of native polyphenols. The use of high-resolution metabolomic techniques has the power to integrate the analysis of systemic polyphenol microbial metabolites, by providing a direct quantitative measure of the compounds that originate from gut microbes and enter circulation in response to polyphenol digestion. Koutsos et al.(Reference Koutsos, Lima and Conterno73) showed an application of the synergic measure of gut microbiota composition and metabolite profile in a colonic fermentation model where whole apples were employed as a growth substrate and 20 % feces were used as inoculum(Reference Koutsos, Lima and Conterno73). The authors observed prebiotic-like effects of whole apples after 24 h anaerobic fermentation (i.e. an increase in Bifidobacterium and Lactobacillus spp), together with an increase of microbial proanthocyanidin metabolites(Reference Koutsos, Lima and Conterno73). Application of metabolite profile analysis to human dietary intervention studies has the capability to find a link between gut microbial metabolism of plant phenolics and their physiological effect in vivo. Recently, Trošt et al.(Reference Trošt, Ulaszewska and Stanstrup74) highlighted how specific blood and urine apple polyphenol metabolites associate with the presence of certain bacterial genera, thus stressing the attention on the importance of a co-metabolic process involving both human and bacterial enzymes to catabolise phenolic compounds(Reference Trošt, Ulaszewska and Stanstrup74).
Bile acids
Primary (1°) BA are synthesised in the liver from cholesterol and released into the small intestine in response to food intake to aid lipid absorption and cholesterol catabolism. Within the gut, they are converted into secondary (2°) BA by the microbiota via deconjugation, dehydrogenation and dehydroxylation. Importantly, this process is regulated by negative feedback through ileal and hepatic nuclear FXR activation by BA. The gut microbiota, therefore, regulates the production of 2° BA via transformation and synthesis of 1° BA via the enterohepatic FXR-fibroblast growth factor 15 axis 1. Recent studies in animal models have shown that probiotic bile salt hydrolase activities(Reference Joyce, MacSharry and Casey75), polyphenols(Reference Chen, Yi and Zhang76) and prebiotic fibres such as oat β-glucans(Reference Drzikova, Dongowski and Gebhardt77, Reference Arora, Loo and Anastasovska78) can modulate the gut microbiota and the profile of BA returning through the enterohepatic circulation (Fig. 2). Indeed, resveratrol, a well-studied polyphenol characteristic of the Mediterranean diet, has recently been shown to reduce production of the cardiotoxicant trimethylamine-N-oxide and associated vascular disease by increasing hepatic BA neosynthesis via gut microbiota modulation of the enterohepatic FXR-fibroblast growth factor 15 axis in mouse models of CVD(Reference Chen, Yi and Zhang76). Studies by Joyce and coworkers have shown the potential of probiotics possessing different bile salt hydrolysing enzymes to impact on the enterohepatic circulation of BAs and regulate metabolic pathways responsible for glucose and lipid metabolism, intestinal integrity, inflammation and circadian rhythm(Reference Joyce, MacSharry and Casey75, Reference Govindarajan, MacSharry and Casey79). In particular, in germ-free laboratory animals, colonisation with one individual bile salt hydrolysing positive bacteria can have a profound impact on the expression of genes related to circadian rhythm, lipid metabolism, intestinal homeostasis and immune function(Reference Joyce, MacSharry and Casey75).
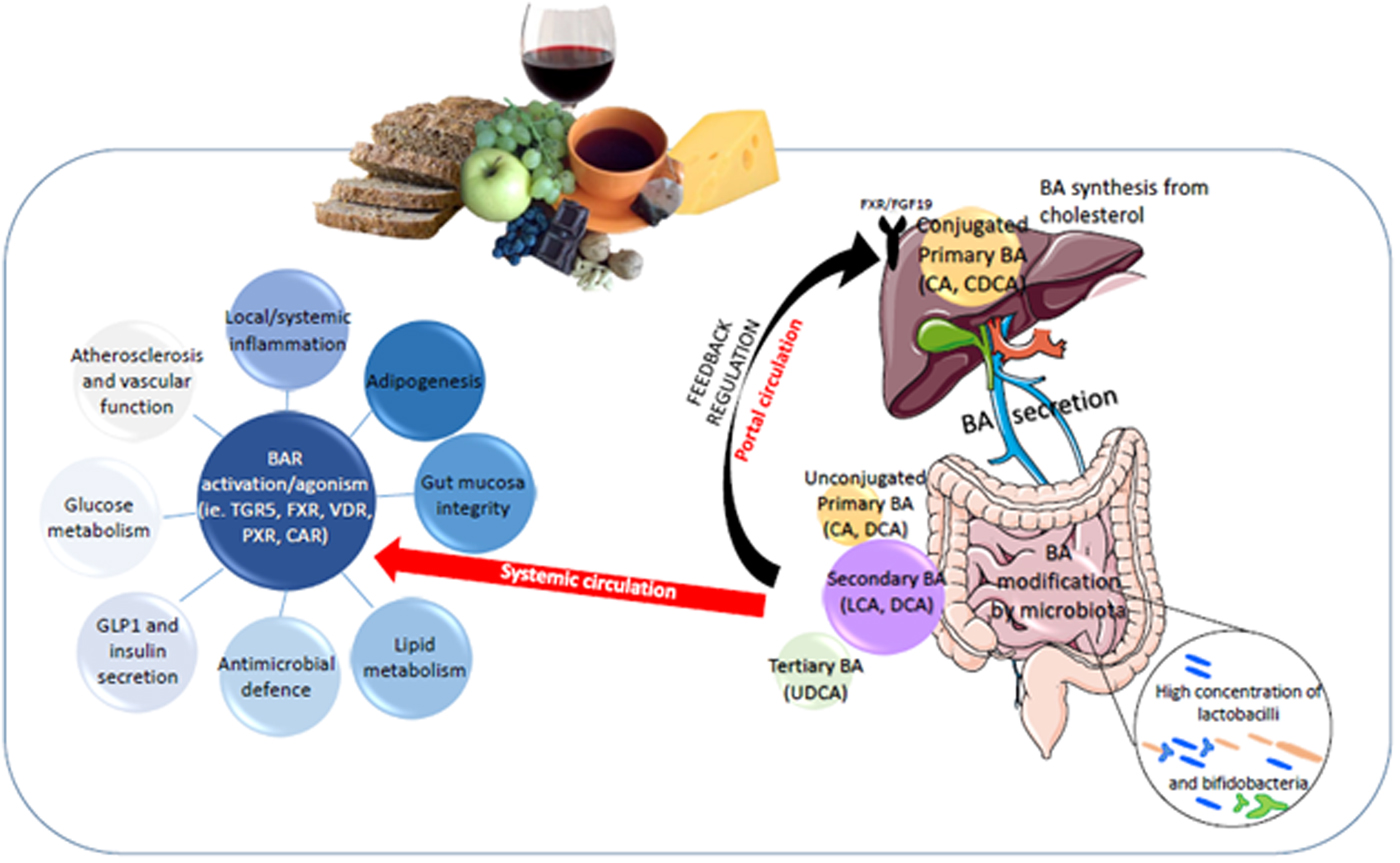
Fig. 2. Bile acid (BA) regulation of metabolic health through diet:gut microbiota interaction. BAR, BA receptor; CA, cholic acid, CDCA, chenodeoxycholic acid; LCA, litocholic acid; DCA, deoxycholic acid; UDCA, ursodeoxycholic acid; CHOL, cholesterol regulation within the liver; PXR, pregnane X receptor; LXR, liver X receptor; CAR,Constitutive Androstane receptor; VDR, Vitamin D receptor.
Similarly, other studies have shown certain fibres (e.g. β-glucans/oats) and complex plant food polyphenols such as the proanthocyanidins or condensed tannins, may sequester BAs, tightly binding them in the small intestine and driving them distally into the colon where they may be excreted or further transformed by the resident microbiota into 2° BA or modified chemical structures. Indeed, BA sequestering agents are recognised pharmaceutical agents of considerable clinical and market importance in treating the metabolic disease as they lower cholesterol, reduce obesity, improve insulin sensitivity and induce thermogenesis(Reference Watanabe, Morimoto and Houten80). Similarly, antibiotic attenuation of the gut microbiota alters GATA4-controlled expression of apical sodium-dependent BA transporter, increasing absorption and decreasing hepatic synthesis of BA(Reference Out, Patankar and Doktorova81). In animal models of obesity, exercise has been confirmed to alter the composition of the gut microbiota and BA metabolism(Reference Meissner, Lombardo and Havinga82, Reference Petriz, Castro and Almeida83). This observation if confirmed in human subjects, would provide powerful support for current healthy diet/healthy life guidelines. Furthermore, BA themselves also have a direct modulatory affect upon the gut microbiota(Reference Devkota, Wang and Musch84), with taurocholate, for example, increasing the relative abundance of bacteria associated with inflammation in animal models of inflammatory bowel disease. Therefore, diet and exercise, by directly modulating the gut microbiota and their role in the enterohepatic circulation of BA have the potential to alter circulating BA profiles and their subsequent ability to regulate host lipid, glucose and xenobiotic metabolism, energy usage/storage and appropriate immune response (Fig. 2). Qualitative and quantitative changes in intestinal and plasma BA profile in response to dietary fibre-induced gut microbiota modulation was shown to positively impact on endothelial function in a murine model for CVD(Reference Catry, Bindels and Tailleux85). The study showed that fructan supplementation induced an increase in Bifidobacterium spp and Akkermansia muciniphila, modulation of BA composition, increase in intestinal endocrine L-cells and glucagon-like peptide-1 production, all acting on the nitric oxide synthase/nitric oxide pathway. However, we currently lack direct evidence in human subjects that foods, diets and life-style interventions which modulate the gut microbiota also modulate circulating BA profiles and influence cardio-metabolic health. Similarly, because CBA have rarely been measured in nutritional interventions designed to assess the efficacy of foods/diets to reduce the risk of chronic diet-associated disease, the mechanistic role of modified BA signalling in improving host health, despite demonstration in animal studies, has yet to be demonstrated clearly in at-risk human populations. The project entitled CABALA_DIET&HEALTH(Reference Cabala86) is a current research project designed to specifically address these shortcomings and aims to establish circulating BA profiles as biomarkers of metabolic health, modulated by diet and exercise.
Bariatric surgery is currently the most effective therapy for morbid obesity, and indeed obesity-associated diabetes often goes into remission rapidly following gastrointestinal re-modelling(Reference Mingrone, Panunzi and De Gaetano87, Reference Schauer, Mingrone and Ikramuddin88). Recent studies have suggested that early changes in BA metabolism, particularly increases in ursodeoxycholic acid (UDCA) and its glycine and taurine conjugates in the first month after surgery act as insulin-sensitising agents(Reference Albaugh, Flynn and Cai89). Work by others shows that deoxycholic acid (DCA) is characteristic of obesity(Reference Yoshimoto, Loo and Atarashi90). Transformation of conjugated cholic acid into DCA by the colonic microbiota has been shown to determine the percentage of DCA in blood(Reference Thomas, Veysey and French91). Importantly, both UDCA and DCA, as secondary BA, are produced by the gut microbiota, respond to changes in diet in animal models and are closely linked to profiles of gut bacteria and their bile salt hydrolysing and 7α/β-hydroxylation capabilities. They also show differential abilities to activate FXR and TGR5, two key BA receptors responsible for lipid, glucose, BA and immune homeostasis. Indeed, oral feeding of UDCA to non-diabetic human subjects lowers blood postprandial glucose levels and increases secretion of glucagon-like peptide-1, an important incretin involved in regulating glucose-induced insulin secretion, in addition to controlling gastric emptying(Reference Murakami, Une and Nishizawa92). In rats, UDCA ingestion ameliorates fructose-induced metabolic syndrome(Reference Mahmoud and Elshazly93). Similarly, rectal delivery of BA increases satiety and reduces food intake by stimulating glucagon-like peptide-1 and PYY(Reference Wu, Li and Liu94). UDCA, therefore with low agonist potential for the G protein-coupled BA receptor and FXR, is amongst the more promising modulators of insulin/glucose homeostasis, steroid and lipid/BA metabolism, intestinal function and inflammation pathways directly regulated by these receptor molecules, suggesting competitive exclusion or synergistic signals may play an important role here.
Animal studies have shown that energy restriction diets, associated with longevity and low incidence of chronic diet associated disease, can radically modulate circulating BAs both in quantity (increasing total serum BA by 162 %) and in profile(Reference Fu and Klaassen95). Similarly, oral ingestion of UDCA decreases age-related adiposity and inflammation in mice(Reference Oh, Bae and Lee96). Cheng et al.(Reference Cheng, Larson and McCabe97) using LC/MS quantification of 217 plasma metabolites in 647 individuals followed up after 20 years found that the BA taurocholate was associated with lower odds of longevity (reaching the age of 80 years), although apparently not through CVD or cancer incidence. However, they noted that current lack of understanding of factors governing circulating BA concentrations limited the usefulness of taurocholic acid (TCA) as a marker of overall BA activity(Reference Cheng, Larson and McCabe97). Profiles or ratios of circulating BA have been suggested to reflect the risk of colorectal cancer and to be sufficiently reproducible for epidemiological studies(Reference Costarelli, Key and Appleby98). More recently, Ho et al.(Reference Ho, Larson and Ghorbani99) using targeted metabolomics of plasma from 2383 Framingham Offspring cohort participants, found that circulating BA profiles were strongly associated with insulin resistance in obese but not lean individuals(Reference Ho, Larson and Ghorbani99). Using 183 metabolites identified from untargeted MS-based metabolomics, Alonso et al.(Reference Alonso, Yu and Qureshi100) identified two conjugated CBA (glycolithocholate sulfate and glycocholenate sulfate) as significant biomarkers of CVD risk in 1919 African-American participants of the Atherosclerosis Risk in Communities study but concluded that further study and replication were needed(Reference Alonso, Yu and Qureshi100).
CBA profiles are therefore emerging as important signalling molecules intricately involved in mammalian cholesterol and lipid metabolism, glucose homeostasis, thermogenesis, inflammation and intestinal function. Diet:microbiota interactions in the gut are likely to play a key role in influencing both hepatic BA neosynthesis and production of 2° BA in the intestine. Dietary change has been shown to alter both faecal BA profiles and the ability of the gut microbiota to generate 2° BA(Reference O'Keefe, Li and Lahti21) but we still know little about how diet:microbe interactions in humans regulates CBA profiles. Human dietary intervention studies could highlight the link between BA profiles and metabotype with gut microbiota signatures in order to highlight the molecular basis of BA regulation of immune and metabolic homeostasis.
Conclusions
The interest in studying the gut microbiota has enormously increased over the past decade, especially since the scientific community has recognised the key role of intestinal microbial commensals in multiple physiological functions of the human organism. Particularly the gut microbiota has been identified as an important metabolic organ that provides us with important pathways, which directly have an impact on the way the human body bio-transforms dietary components. Hence current nutrition intervention studies looking at the impact of diet on human health and disease risk include monitoring of gastrointestinal microbial members and their metabolic input, alongside measures of clinical parameters. Despite the amount of data that can be generated by high-throughput microbial molecular techniques, mining information on the role of thousands of bacterial populations inhabiting the human gut still remains a challenge. Studies on extreme animal and human phenotypes or pathological states have allowed advances in this field, by providing patterns of association between bacterial groups and certain concomitant systemic features. However, the topic remains wide and often clear considerations regarding the function of resident taxonomic groups cannot be easily drawn and the etiological contribution of gut microbial populations to human health and disease is still uncertain. Nevertheless, progress has been made on the causative link between gut microbiota and health, especially thanks to intervention studies that monitored changes in microbial composition and activities in response to a specific dietary challenge.
Acknowledgements
The authors would like to thank the Nutrition Society for inviting Francesca Fava to present at the Irish Section Meeting 2018 at the University of Ulster, Coleraine, Northern Ireland (U. K.).
Financial support
This study was funded in part by Accordo di Programma between Fondazione Edmund Mach (San Michele all'Adige, Italy) and Autonomous Province of Trento (Trento, Italy); in part by the ERA JPI HDHL action project ‘CABALA_DIETH&HEALTH. CirculAting Bile Acids as biomarkers of metabolic Health_Linking microbiotA, diet and Health’ (grant number 696295, http://www.cabalaproject.eu/; http://www.healthydietforhealthylife.eu/index.php/64-opencalls/ 309-cabala-diet-health).
Conflict of interest
None.
Authorship
FF and KT wrote the paper; LR drew the figures and critically read the paper.