Base excision repair (BER) is the primary mechanism used to fix oxidative damage to DNA. However BER efficiency declines with age( Reference Intano, Cho and McMahan 1 , Reference Swain and Rao2 ). To determine whether epigenetic events contribute to the ageing process through deregulation of BER gene expression we quantified DNA methylation and histone acetylation at BER-gene promoters (Ogg1 and Apex) and BER repair activity in ageing and dietary restricted (DR) mice.
We measured promoter methylation by pyrosequencing in brain and livers from ad libitum (AL) and 40% DR mice at 3, 12, 24 and 30 months of age (n=5–7/group). Ogg1 promoter methylation decreased with age in the liver (p=0.018) and brain (p=0.016) and DR reduced Ogg1 methylation (p=0.014) in the brain. At 30 mo, we observed a 2.5 fold enrichment in histone 4 acetylation as measured by the ChIP assay in liver Ogg1 promoter (p=0.004) and a 2 fold enrichment at Ogg1 (p=0.02) and Apex promoters (p=0.031) in the brain. Ogg1 expression in the liver decreased by 40% with age and DR (p=0.0031) and with DR only in the brain (p=0.002). Apex expression did not change with age but was lower in DR animals (p=0.003). A comet-based in vitro assay for BER incision activity( Reference Langie, Cameron and Waldron 3 ) revealed no significant changes in either tissue. 8-oxoguanine lesions measured by HPLC-ECD decreased with age (p<0.001) in the liver but not in the brain.
Table 1. Summary of results from the liver
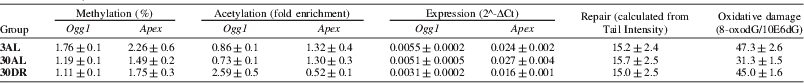
Table 2. Summary of results from the brain

In summary, our data suggest that epigenetic processes may contribute to transcriptional changes in BER-related genes during ageing and with DR.
This work was funded by the Research Councils through the Lifelong Health and Wellbeing Initiative.




