Cereal grains belong to wide variation of food cultures forming familiar staple foods from the early ages of history until recent years. Cereal grains are major sources of energy, carbohydrates and protein sources globally(Reference McKevith1). However, cereal grains have been neglected as a relevant and culturally familiar source of dietary plant protein. As such they provide feasible plant foods and, from the sustainability viewpoint, assist the need for transition from animal-based diets towards plant-based diets. In their whole grain form, all parts of the cereal grains are retained and as such they are also rich in many vitamins and minerals and bioactive compounds such as phytochemicals and fermentable and non-fermentable fibres delivering many health benefits(Reference Poutanen, Kårlund and Gómez-Gallego2). Indeed, whole grain consumption is associated with decreased risk of multiple diseases and mortality, such as CVD(Reference Barrett, Batterham and Ray3,Reference Tieri, Ghelfi and Vitale4) , type 2 diabetes(Reference Barrett, Batterham and Ray3–Reference McRae5), hypertension(Reference Dorosti, Heidarloo and Bakhshimoghaddam6–Reference Kashino, Eguchi and Miki8) and some cancers such as colorectal cancer(Reference Tieri, Ghelfi and Vitale4,Reference McRae5,Reference Reynolds, Mann and Cummings7) . Conversely, a diet low in whole grains has been identified as one of the highest-ranked dietary risk factors for burden of diseases globally(Reference Afshin, Sur and Fay9). Nevertheless, precise mechanisms behind these health impacts are not well known. Because of the observed health benefits, whole grain consumption is encouraged in dietary guidelines worldwide(Reference Seal, Nugent and Tee10,Reference Miller11) . That been said, the average consumption of whole grains in populations fails to meet the recommendations(Reference Micha, Khatibzadeh and Shi12,Reference van der Kamp, Jones and Miller13) . Recently, Nordic Nutrition recommendations(14) recommended consuming at least 90 g/d of whole grain consumption with the preference of other grain cereals than rice. However, there is no global consensus on the recommended amount or the number of portions of whole grain products to be consumed daily, and the recommendations vary from qualitative to quantitative statements(Reference Miller11,Reference van der Kamp, Jones and Miller13) . This may make the shift to consume more whole grain foods even more challenging.
Until recently the consensus on the definition for whole grain cereal foods has been missing. This has been a challenge in practical terms in consumer understanding, but also when evaluating and comparing previous scientific reports on the health impact of cereal grains. Other grains, such as pulses, legumes and oilseeds, are not included in any definitions nor in dietary recommendations for whole grains as they differ substantially from cereal grains in their anatomy and composition(Reference Seal, Nugent and Tee10). Consequently, our focus in this review is exclusively on whole grain cereals. Consensus on global definition of whole grains was published by a working group of Whole Grain Initiative(Reference van der Kamp, Jones and Miller13). According to this consensus, ‘whole grains shall consist of the intact, ground, cracked, flaked, or otherwise processed kernel after the removal of inedible parts such as the hull and husk. All anatomical components, including the endosperm, germ, and bran must be present in the same relative proportions as in the intact kernel’. Moreover, the whole grain foods have definition of ‘A whole-grain food shall contain at least 50% whole-grain ingredients based on dry weight. Foods containing 25–50% whole-grain ingredients based on dry weight, may make a front-of-pack claim on the presence of whole grain but cannot be designated ‘whole grain’ in the product name’. The definitions have been ratified by the leading international cereal science associations, including the C & A Association, the HealthGrain Forum and the International Association for Cereal Science and Technology(Reference van der Kamp, Jones and Miller13). Furthermore, the Nordic Nutrition recommendations(14) have adopted a consensus in defining the whole grains, simplifying the harmonisation process across the Nordic countries. However, there are still challenges in implementing these definitions on a global scale, and their adoption in practical usage requires agreement from regulatory bodies as well.
From the intestine-mediated health impact point of view, whole grains are an interesting combination of various fibres and protein which are bound within the plant cell structures. This combination of fibre and protein in cereals is of great interest, for example, how is it processed and metabolised in the gastrointestinal tract? Fibre composition varies greatly among different whole grains(Reference NPV and Joye15). They are rich in both soluble and insoluble dietary fibres, among which some are fermentable, such as β-glucans, pectins, fructans, arabinoxylan and resistant starch and some non-fermentable such as cellulose and lignans, by the intestinal microbiota(Reference O'Grady, O'Connor and Shanahan16). Latter division is partly mixed and overlapping with concepts of fibre being soluble or not. It is currently understood that dietary fibres that are fermentable by the intestinal microbiota induce the increase in the intestinal microbiota diversity and support the growth of bacterial species associated with health beneficial metabolites such as SCFA. Especially the non-fermentable fibre may possess these beneficial impacts on the intestinal microbiota, allowing the adherence of specific bacteria and facilitating the fermentation of the fermentable fibre(Reference Liu, Liu and Vera17,Reference Li, Zhao and Li18) . Most cereal grain proteins are located in the endosperm, but the aleurone and subaleurone layers of the bran have the highest protein content(Reference Shewry and Halford19). Fibre and other plant tissue structures have been demonstrated to reduce protein digestibility in both in vitro and human trials, but this effect might differ between individuals and also depend on the type of fibre(20). Due to lower bioavailability of the cereal protein, part of it reaches the colon within the fibre fraction. Processing during the food manufacturing causes changes in grain constituents, which may have an impact on the digestibility and bioavailability of protein(Reference Poutanen, Kårlund and Gómez-Gallego2). To this end, there is the question of what happens to fibre fraction bound or otherwise non-absorbed cereal protein in the colon?
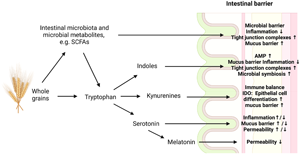
Our current understanding is mainly based on animal protein fermentation in the colon. Colonic bacteria favour carbohydrate fermentation over proteins, resulting in proteins being fermented mostly in the distal colon(Reference Korpela21). Proteolytic fermentation in the distal colon yields metabolites, such as ammonia, certain phenols and branched-chain amino acids that are usually regarded as harmful for the intestinal barrier function and may activate pro-inflammatory mechanisms in the intestine, while also predisposing the individual to non-communicable diseases through systemic effects(Reference Canfora, Meex and Venema22). However, it can be assumed that the fermentation activity of dietary fibre in the proximal colon influences the activity of the microbiota, promotes the production of beneficial metabolites and strengthens the intestinal barrier function, thereby promoting beneficial health outcomes that may counterbalance the potential harm of proteolytic activity. In addition to SCFA, cereal fibre fermentation produced many other metabolites(Reference Desai, Seekatz and Koropatkin23–Reference Blaak, Canfora and Theis25) including derivatives of fibre-embedded phytochemicals that have been associated with health-supporting effects. Moreover, protein content differs between different grains(Reference Poutanen, Kårlund and Gómez-Gallego2). The metabolically active cereal proteins include mostly enzymes and storage proteins such as albumins and globulins(Reference Jahan-Mihan, Luhovyy and Khoury26). Some cereal proteins, such as gluten, and peptides, such as prolamins, may trigger immune system and cause symptoms for some individuals(Reference Poutanen, Kårlund and Gómez-Gallego2). Previous reports, both from the others and our own studies, have shown that the intestinal microbiota-produced metabolites have great importance for the overall health impact of the whole grain cereals. Novel research findings link tryptophan, an important amino acid within the cereal protein fraction, with health and risk of disease. This review aims to investigate the relationship between the health impact of whole grains mediated via the interaction with intestinal microbiota and intestinal barrier function with special interest on tryptophan metabolism, focusing on the role of the intestinal microbiota and their impact on barrier function.
Whole grains and intestinal microbes
One potential link between whole grains and their health effects is the impact of whole grains on intestinal microbiota. Consuming various types of whole grains can lead to the growth of different microbiota species, which in turn leads to the production of diverse metabolites, including those derived from tryptophan metabolism(Reference Kundi, Lee and Pihlajamäki27). However, despite the efforts to evaluate the impact of whole grains on intestinal microbiota composition, the results remain inconclusive and vary among different studies(Reference Koecher, McKeown and Sawicki28). This has been pointed in a recent review by Koecher et al., making difficult to draw any conclusions on the effect of whole grains on the intestinal microbiota because there are no consistent effects, even when grouping studies evaluating the same grains and using similar microbial measurement techniques(Reference Koecher, McKeown and Sawicki28). We agree with Koecher et al. that these differences are likely attributed to variations in the understanding of what constitutes whole grains (lack of consistent use of whole grain definition), variations in the quantity of whole grains tested, diverse techniques employed for microbiota determination, lack of control in dietary intake in some of the studies and the interindividual differences in microbiota composition and response, which diminish the statistical power of most of the studies(Reference Koecher, McKeown and Sawicki28,Reference Deehan, Duar and Armet29) . Then there is a need for future studies to clarify the impact of whole grains in microbiota composition. Conversely, whole grains modulate microbiota activity to produce bioactive compounds that may exert a physiological effect. Fibre from whole grains is fermented by intestinal microbiota. This fermentation yields SCFA, including butyrate, acetate and propionate and other metabolites that have been associated with several health impacts(Reference Poutanen, Kårlund and Gómez-Gallego2). The consumption of diet containing whole grains is associated with higher levels of total SCFA and acetate(Reference Vanegas, Meydani and Barnett30,Reference Vanegas, Meydani and Barnett31) . Notably, the daily consumption of oat and barley β-glucan has been shown to increase the concentration of SCFA in faeces of the subjects(Reference De Angelis, Montemurno and Vannini32). The consumption of rye products has been associated with increased butyrate-producing bacteria and higher levels of butyrate in plasma(Reference Iversen, Dicksved and Zoki33). Additionally, the postprandial effect on butyrate concentrations, as well as propionate concentrations, in plasma has been observed after the consumption of rye bread(Reference Lappi, Mykkänen and Knudsen34).
In addition to fibre, whole grains contain other compound that can influence microbiota composition and activity such as polyphenols, sterols, tocols and betaine(Reference Gong, Cao and Chi35). The mechanisms of the effects of these other compounds from whole grains are understudied in human subjects. Polyphenols seem to stimulate the production of SCFA and other organic acids(Reference Gong, Cao and Chi35,Reference Bié, Sepodes and Fernandes36) and studies in vitro and in animal models associate betaine with an increase of SCFA-producing bacteria(Reference Koistinen, Kärkkäinen and Borewicz37,Reference Du, Zhang and Luo38) . Nonetheless, the specific mechanism of action and contribution to health in human subjects remain unclear.
Intestinal barrier
Intestinal barrier is a dynamic entity that consists of multiple elements. It consists of (1) microbial barrier that is composed of commensal bacteria and chemical barrier composed of gastrointestinal secretions in the lumen, (2) the microclimate including the unstirred water layer, glycocalyx and mucus layer, (3) the epithelium constituted of a single layer of different cell types, such as enterocytes, Paneth cells and goblet cells, that are connected to each other by apical junctional complexes, (4) the immunological barrier including a variety of immune cells and (5) the lamina propria(Reference Camilleri39–Reference Fernandez-Cantos, Garcia-Morena and Iannone41). There are numerous ways the intestinal barrier protect host from the harmful substances and pathogens. For example, mucus serves as the first physical defence in the barrier by containing several immune factors and thus protecting the epithelial cells from the direct contact with antigens, toxins and pathogenic bacteria(Reference Camilleri39–Reference Régnier, Van Hul and Knauf42). The epithelial cells (enterocytes and Paneth cells) react to noxious stimuli by secretion of antimicrobial peptides and chloride (Reference Camilleri39,Reference Schoultz and Keita40,Reference Takiishi, Fenero and Câmara43) . In addition, junction complexes regulate intercellular transport, including blocking the entry of pathogens(Reference Camilleri39,Reference Fernandez-Cantos, Garcia-Morena and Iannone41,Reference Takiishi, Fenero and Câmara43) . The lamina propria provides defence based on innate and acquired immunity cells as well as endocrine and secretomotor mechanisms(Reference Camilleri39,Reference Schoultz and Keita40) .
The dysfunction of the intestinal barrier, also referred as ‘leaky gut’, is characterised by an increase in intestinal permeability. This condition can be induced by sustained inflammation or infections(Reference Schoultz and Keita40,Reference Takiishi, Fenero and Câmara43) . As a result of increased intestinal permeability, pathogens and lipopolysaccharides (LPS) are able to pass through the intestinal barrier, triggering the production of proinflammatory cytokines. These cytokines can induce systemic proinflammatory immune responses(Reference Houser and Tansey44) and alter the structure of tight junctions, thereby further disrupting intestinal permeability(Reference Yang, Chu and Gao45,Reference Shehata, Yalçın and Latorre46) . An example illustrating the impact of altered intestinal homeostasis is the disturbance in the production and secretion of endocrine hormones, that subsequently trigger metabolic diseases(Reference Régnier, Van Hul and Knauf42). Thus, maintaining the integrity of the intestinal barrier serves as a beneficial target in promoting overall health. Tryptophan and its metabolites may play a role in supporting this essential function of the intestinal barrier by influencing on intestinal permeability, mucus production, immune balance and intestinal microbe composition that will be discussed in following sections.
Tryptophan metabolism in the intestine
Amino acids, peptides and proteins that are attached to cereal structures, such as the fibre fractions, and are not absorbed in the small intestine ultimately reach the large intestine, where some of them undergo fermentation by the intestinal microbiota(Reference Marx, Lane and Hockey50). After being released from the cereal structures, proteins are broken down into smaller peptides and amino acids by proteases and peptidases produced by bacteria in the large intestine. Among the other amino acids, tryptophan can participate in various metabolic pathways, especially those induced by colonic microbes(Reference Gao, Mu and Farzi49,Reference Agus, Planchais and Sokol51,Reference Taleb52) .
The majority of dietary tryptophan, such as other amino acids, is absorbed from the small intestine(Reference Erickson and Kim47). In the epithelial cells, tryptophan is released into the peripheral circulation, or it can be degraded to different metabolites by the enzymes of the intestinal cells(Reference Roth, Zadeh and Vekariya48). Microbial activity could impact tryptophan availability and metabolism in the small intestine, but the current evidence is very limited and mostly focused on the colonic microbiota. Several common groups of intestinal bacteria including Lactobacillus, Streptococcus and Escherichia coli among others have been reported to express tryptophan synthetase(Reference Gao, Mu and Farzi49) and potentially may contribute to tryptophan production. Future studies clarifying the role of the small intestinal microbiota in the tryptophan metabolism may be needed.
Tryptophan has three major metabolic pathways: kynurenine, serotonin and indole pathways (Fig. 1).

Fig. 1. Simplified illustration depicting tryptophan metabolism in the intestine. The main pathways of tryptophan metabolism are kynurenine pathway, serotonin pathway and indole pathway. Additionally, kynurenine pathway is active in immune cells and serotonin pathway is active in serotonergic neurons (not shown). The production of indole derivatives is attributed to the intestinal microbiota. 5-HTP, 5-hydroxytryptophan; EC, enterochromaffin; IA, indole-3-acrylic acid; IAA, indole-3-acetic acid; IAAld, indole-3-acetaldehyde; IAld, indole-3-aldehyde; IPA, indole-3-propionic acid; KYNA, kynurenic acid; QUIN, quinolinic acid.
Kynurenine pathway
Kynurenine pathway covers a considerable part of the entire tryptophan metabolism in the gastrointestinal tract(Reference Taleb52). Tryptophan is degraded to kynurenine in the intestinal epithelial cells and in the immune cells by the enzyme indoleamine-2,3-dioxygenase 1 (IDO1), whose activity is induced by the inflammatory signalling(Reference Cervenka, Agudelo and Ruas53). Kynurenine can be further metabolised through kynurenic acid or quinolinic acid pathways, which produce multiple derivatives affecting health and disease(Reference Cervenka, Agudelo and Ruas53,Reference Kennedy, Cryan and Dinan54) .
Indole pathway
The indole pathway is another primary metabolic route for tryptophan, constituting a small percentage of intestinal metabolism(Reference Taleb52). Through the catalytic action of tryptophanase and decarboxylase enzymes, tryptophan can undergo modifications within the indole pathway, resulting in the production of diverse compounds called indole derivatives(Reference Li, Zhang and Hu55,Reference Ye, Li and Anjum56) . In contrast to serotonin and kynurenine, indole derivatives are almost exclusively synthesised through the metabolism of the intestinal microbiota(Reference Gao, Mu and Farzi49,Reference Li, Zhang and Hu55,Reference Gheorghe, Martin and Manriquez57) , and multiple species have been reported to produce these metabolites(Reference Roager and Licht58).
Serotonin pathway
A small portion of the tryptophan obtained from food is metabolised into serotonin within the large intestine. Interestingly, this contribution accounts for a significant proportion, estimated to be some 90 % of the body's overall serotonin production(Reference Gheorghe, Martin and Manriquez57). The synthesis of serotonin in the intestine occurs in enterochromaffin cells, a specific type of enteroendocrine cells, as well as in serotonergic neurons of the enteric nervous system(Reference Layunta, Buey and Mesonero59). Within the intestine, the enzyme tryptophan hydroxylase plays a crucial role as a limiting factor in the production of serotonin, catalysing the conversion of tryptophan to 5-hydroxytryptophan(Reference Bertrand and Bertrand60,Reference O'Mahony, Clarke and Borre61) . Subsequently, 5-hydroxytryptophan is further converted to serotonin through the action of aromatic amino acid decarboxylase.
Whole grains and tryptophan metabolism
The relationship between the consumption of whole grains and the modulation of tryptophan metabolism has been investigated in human studies, although to a limited extent. In healthy adults, it was found that plasma tryptophan concentrations decreased after the consumption of whole grains(Reference Navarro, Tarkhan and Shojaie62). However, this effect was not observed in postmenopausal women with dyslipidaemia(Reference Lankinen, Schwab and Seppänen-Laakso63). Several studies have demonstrated that the consumption of whole grains is associated with the suppressed activity of the kynurenine pathway(Reference Navarro, Tarkhan and Shojaie62,Reference Zhu, Wang and Sha64,Reference Qi, Li and Yu65) . Moreover, there is a direct association between the consumption of whole grains and higher fibre intake with serum indole-3-propionic acid (IPA)(Reference Qi, Li and Yu65,Reference de Mello, Paananen and Lindström66) . The relationship with other indole derivatives varies, with some studies showing an inverse association(Reference Qi, Li and Yu65) and others showing a positive association(Reference Lankinen, Schwab and Seppänen-Laakso63,Reference Garcia-Aloy, Rabassa and Casas-Agustench67) , depending on the specific indole derivative. Additionally, partial effects on serotonin metabolism have been observed, with some studies reporting an inverse association(Reference Navarro, Tarkhan and Shojaie62,Reference Zhu, Wang and Sha64,Reference Keski-Rahkonen, Kolehmainen and Lappi68) and one study showing a positive association(Reference Qi, Li and Yu65) between whole grain consumption and serotonin concentrations. Establishing causality in this relationship, however, is challenging, and there are still limited studies available to definitively determine the direction of these associations.
Whole grains can exert an influence on tryptophan metabolism through interactions with the intestinal microbiota, and the presence of fibre in whole grains plays a notable role in establishing this connection. Certain fibre types have been associated with the production of IPA by specific bacterial genera found in faecal samples(Reference Qi, Li and Yu65). Various phyla and genera of intestinal bacteria have been found to employ tryptophan metabolic pathways within the intestine(Reference Roager and Licht58,Reference Daisley, Koenig and Engelbrecht69,Reference Kaur, Bose and Mande70) . Different genera may possess the ability to utilise distinct metabolic pathways for tryptophan(Reference Roager and Licht58,Reference Qi, Li and Yu65,Reference Kaur, Bose and Mande70) , which could exert a significant influence on the tryptophan metabolism in the host. The absence or depletion of the intestinal microbiota in mice has been found to decrease the activity of the kynurenine pathway, resulting in decreased kynurenine levels and increased tryptophan concentrations in the bloodstream(Reference Wikoff, Anfora and Liu71–Reference Desbonnet, Clarke and Traplin73). In addition, previous studies conducted on germ-free mice have indicated disruptions in serotonin synthesis(Reference Wikoff, Anfora and Liu71,Reference Clarke, Grenham and Scully72,Reference Hata, Asano and Yoshihara74,Reference Engevik, Luck and Visuthranukul75) .
The impact of intestinal microbiota on tryptophan metabolism can be attributed to microbial products. Among these products, SCFA are noteworthy. In vitro studies have demonstrated that SCFA can enhance intestinal serotonin metabolism by increasing the expression of the tryptophan hydroxylase 1 enzyme(Reference Reigstad, Salmonson and Rainey76,Reference Yano, Yu and Donaldson77) . However, in mice, propionate has shown an inverse correlation as well(Reference Kundi, Lee and Pihlajamäki27). SCFA also have been observed to stimulate serotonin release from enterochromaffin cells in rats(Reference Fukumoto, Tatewaki and Yamada78), but this effect has not been replicated in vitro (Reference Martin, Lumsden and Young79). Furthermore, butyrate has been found to reduce the expression of the IDO1 enzyme in the intestine, leading to a decrease in the conversion of tryptophan into kynurenine(Reference Martin-Gallausiaux, Marinelli and Blottière80). In addition to SCFA, another microbial product called deoxycholate, which is a secondary bile acid produced through microbial biotransformation, has been shown to stimulate serotonin release from enterochromaffin cells(Reference Yano, Yu and Donaldson77). Furthermore, alterations in the microbiota can interfere with the functioning of serotonin transporter proteins, resulting in increased concentrations of serotonin(Reference Gao, Xiong and Grabauskas81).
Tryptophan metabolism can be influenced by the intestinal microbiota through immune responses as well. The microbiota plays a role in regulating the immune response through Toll-like receptors(Reference Rakoff-Nahoum, Paglino and Eslami-Varzaneh82,Reference Round, Lee and Li83) , and previous studies have associated their activation with increased activation of kynurenine pathway in immune cells(Reference Opitz, Litzenburger and Lutz84,Reference Orhan, Bhat and Sandberg85) . Conversely, alterations in the intestinal microbiome have been associated with changes in intestinal permeability(Reference Godos, Currenti and Angelino86,Reference Martins, Braga Tibães and Sanches87) . Consequently, microbes and their metabolites, which would not typically enter the body through normal routes, gain access, triggering immune cell activation and subsequent inflammation(Reference Levy, Kolodziejczyk and Thaiss88,Reference Lobionda, Sittipo and Kwon89) . This can affect tryptophan metabolism, particularly through the activation of enzymes within the kynurenine pathway.
Conversely, tryptophan has the ability to alter the composition and metabolic activity of the intestinal microbiota. For instance, tryptophan supplementation has been shown to enhance the abundance of SCFA-producing bacteria in LPS-challenged mice(Reference Gao, Yang and Liu90) and in pigs(Reference Liang, Dai and Kou91,Reference Liu, Lu and Sun92) . This has led to higher concentrations of SCFA in the colonic digesta(Reference Liu, Lu and Sun92). Furthermore, indoles play a role in interspecies communication, effectively regulating the microbial composition and characteristics within the gastrointestinal tract(Reference Lee, Wood and Lee93). They promote the proliferation of beneficial bacteria and inhibit the growth of pathogenic bacteria in the intestinal tract(Reference Ye, Li and Anjum56). Meanwhile, IDO1 plays an important role in preserving the intestinal microbial diversity within the intestinal environment(Reference Le Floc'h, Otten and Merlot94). These findings emphasise the bidirectional interaction between tryptophan and the intestinal microbiota.
The composition of the intestinal microbiota and its active metabolic pathways appear to be closely linked to the tryptophan metabolism both in the intestine and within the body. However, it is crucial to exercise caution when generalising the findings from studies conducted on animals or in vitro to human populations. Furthermore, the precise mechanisms underlying this interplay are not fully understood, and several unanswered questions remain. Therefore, further investigation is necessary to unravel the cause-and-effect relationships and underlying mechanisms involved in these connections in the context of human biology.
Whole grains, tryptophan metabolism and intestinal barrier
Whole grains and intestinal barrier
The impact of whole grains on intestinal barrier function is closely related to their effects on the intestinal microbiota. Firstly, commensal luminal bacteria inhibit the colonisation of pathogens via numerous mechanisms, such as the production of antimicrobial peptides, pH modification of the lumen content and nutrient competition(Reference Schoultz and Keita40,Reference Pickard, Zeng and Caruso95) . Secondly, the intestinal microbiota has been implicated in modulating the integrity of the intestinal barrier, as it plays a role in regulating epithelium formation(Reference Willing and Van Kessel96) and enhancing the mucus layer through its influence on mucus properties and mucosal immunity(Reference Shi, Li and Duan97–Reference Paone and Cani99). Similarly, a diet lacking in fibre has been shown to erode the mucus layer and promote mucus penetrability in mice(Reference Desai, Seekatz and Koropatkin23,Reference Schroeder, Birchenough and Ståhlman100,Reference Neumann, Steimle and Grant101) . This effect could potentially be attributed to the intestinal microbiota utilising mucus glycoproteins as a source of nutrients(Reference Desai, Seekatz and Koropatkin23).
The connection between whole grains, intestinal microbiota and intestinal barrier function may be mediated through SCFA(Reference Camilleri39,Reference Takiishi, Fenero and Câmara43) . SCFA produced in the fermentation of whole grain fibres are responsible for causing a decrease in pH within the intestinal environment, which in turn supports the microbial barrier(Reference Wong, de Souza and Kendall102). Furthermore, SCFA and particularly butyrate plays a crucial role in supporting the intestinal epithelial cells by serving as important energy sources and possessing anti-inflammatory properties(Reference Schoultz and Keita40,Reference Fernandez-Cantos, Garcia-Morena and Iannone41,Reference Takiishi, Fenero and Câmara43,Reference Hamer, Jonkers and Venema103) . SCFA correlated negatively with the expression of pro-inflammatory cytokine genes in LPS-challenged pigs(Reference Liu, Lu and Sun92). In addition, SCFA have been shown to activate transmembrane G protein-coupled receptors, which take part in regulating gastrointestinal homeostasis and intestinal immunity(Reference Gao, Xu and Liu104). These qualities make SCFA essential for maintaining homeostasis in the intestinal epithelium.
SCFA play a role in regulating the growth and differentiation of epithelial cells(Reference Wong, de Souza and Kendall102,Reference O'Keefe105) . Additionally, they are directly involved in the regulation of intestinal permeability, potentially by accelerating the assembly of tight junctions(Reference Kundi, Lee and Pihlajamäki27,Reference Liu, Lu and Sun92,Reference Suzuki, Yoshida and Hara106–Reference Mariadason, Barkla and Gibson108) . Notably, butyrate has been found to promote the aggregation of tight junctions(Reference Peng, Li and Green107) and enhance the expression of tight junction complex proteins in vitro studies(Reference Zheng, Kelly and Battista109,Reference Ma, Fan and Li110) . In mice, the administration of oat and rye bran resulted in increased mRNA expression of tight junction proteins, which correlated with the presence of SCFA, particularly propionate and butyrate(Reference Kundi, Lee and Pihlajamäki27). Moreover, a positive association was observed between SCFA and occludin mRNA expression in LPS-challenged pigs(Reference Liu, Lu and Sun92).
SCFA are thought to provide support to the intestinal barrier through their effects on the mucus layer as well. In vitro studies have demonstrated that propionate and acetate can stimulate mucin 2 expression(Reference Willemsen, Koetsier and van Deventer111), and propionate has also been associated with colonic mucin levels in mice(Reference Kundi, Lee and Pihlajamäki27). Butyrate has been involved in supporting the mucus barrier by stimulating the production of mucin both in vitro (Reference Burger-van Paassen, Vincent and Puiman112–Reference Liu, Yu and Tian114) and in the colon of mice(Reference Kundi, Lee and Pihlajamäki27). However, it is noteworthy that the transcription of mucin was decreased at higher concentrations(Reference Nielsen, Jensen and Theil113). Interestingly, Gaudier et al. (Reference Gaudier, Rival and Buisine115) discovered that despite the increased expression of the mucin 2 gene, high concentrations of butyrate led to a reduction in the thickness of the adherent mucus layer in mice. Furthermore, higher concentrations of butyrate have been shown to be involved in the disruption of the intestinal barrier associated with cell apoptosis in vitro (Reference Peng, He and Chen116). These findings emphasise the importance of maintaining an adequate production of SCFA, particularly butyrate, in the intestine. By promoting the growth of SCFA-producing bacteria, including whole grains in the diet has the potential to facilitate this adequate production and support intestinal health.
Tryptophan metabolism and intestinal barrier
Tryptophan in the whole grains and tryptophan metabolism within the colon represent additional potential links connecting whole grains, intestinal microbiota and the intestinal barrier function. Several studies have proposed that specific tryptophan metabolites activate the aryl hydrocarbon receptor (AHR), which plays a role in maintaining the integrity of the intestinal barrier(Reference Gao, Xu and Liu104,Reference Zelante, Iannitti and Cunha117,Reference Lamas, Natividad and Sokol118) . Indeed, tryptophan, multiple indole derivatives, 5-hydroxytryptophan and kynurenines have been seen to induce the expression of the AHR genes in vivo and in vitro (Reference Gao, Xu and Liu104,Reference Liu, Yu and Tian114,Reference Hubbard, Murray and Perdew119–Reference Dong, Hao and Murray121) . The stimulating effect on AHR activity varies depending on the specific metabolite(Reference Sridharan, Choi and Klemashevich122). Another receptor implicated in the maintenance of intestinal barrier functions is the pregnane X receptor (PXR). It has been proposed as a key regulator of intestinal barrier function(Reference Venkatesh, Mukherjee and Wang123). Activation of PXR has been shown to have protective effects on the intestinal barrier during inflammation(Reference Garg, Zhao and Erickson124), and PXR is involved in the upregulation of the tight junction complex proteins(Reference Zhao, Zhou and Liang125). It is important to note that the sensitivity of AHR and PXR for tryptophan metabolites differ between human subjects and other mammals(Reference Dong, Hao and Murray121,Reference Venkatesh, Mukherjee and Wang123,Reference Hubbard, Murray and Bisson126) . As a result, the outcomes observed in animal studies may not entirely reflect the actual effects on human subjects.
The absence of tryptophan in the diet can potentially compromise the integrity of the intestinal barrier, as suggested by Régnier et al. (Reference Régnier, Van Hul and Knauf42). Additionally, the depletion of microbial tryptophan metabolism pathways has been found to associate with increased intestinal permeability in a cohort study(Reference Leibovitzh, Lee and Xue127). Conversely, tryptophan increased goblet cells, antimicrobial peptides and mucins in the ileum of LPS-challenged mice, resulting in a reduction of damage to the intestinal mucosal barrier(Reference Gao, Yang and Liu90). Tryptophan has also increased the concentrations and expressions of proteins that contribute to tight junction formation in vitro and in pigs(Reference Liu, Lu and Sun92,Reference Liang, Dai and Liu128,Reference Liu, Gu and Wang129) , aligning with a decrease in permeability observed in vitro (Reference Liu, Gu and Wang129).
Indole metabolites have been shown to regulate epithelial cell proliferation and the production of antimicrobial peptides by promoting IL-22 production through AHR activation(Reference Zelante, Iannitti and Cunha117,Reference D'Onofrio, Renga and Puccetti130) . Indole, specifically, has been found to increase the expression of genes involved in fortifying the mucosal barrier and stimulating mucin production, as well as exhibit anti-inflammatory properties in vitro (Reference Bansal, Alaniz and Wood131). Furthermore, likely mediated by the activation of the PXR, indole has been shown to enhance the expression of proteins involved in the formation of junctional complexes, resulting in increased resistance against epithelial damage both in vitro (Reference Bansal, Alaniz and Wood131) and in the colons of germ-free mice(Reference Shimada, Kinoshita and Harada132).
IPA has been observed to activate PXR as well, with greater activation occurring in the presence of indole or indole-3-acetic acid(Reference Venkatesh, Mukherjee and Wang123). However, this activation has not been consistently observed in vitro (Reference Wlodarska, Luo and Kolde120). IPA has demonstrated the ability to reduce intestinal permeability both in vitro (Reference Jennis, Cavanaugh and Leo133,Reference Li, Zhang and Wu134) and in mice studies(Reference Venkatesh, Mukherjee and Wang123,Reference Jennis, Cavanaugh and Leo133) . The changes in permeability observed in vitro align with the increased expression of tight junction proteins(Reference Li, Zhang and Wu134), and the impact on the expression of proteins within the junction complex has been observed in rats as well(Reference Zhao, Xin and Xue135). In addition, IPA has been shown to elevate the concentrations of mucins and goblet cell secretion products in vitro, suggesting its strengthening effects on the mucus barrier(Reference Li, Zhang and Wu134). Moreover, IPA has displayed anti-inflammatory properties by reducing proinflammatory cytokines and promoting anti-inflammatory cytokine production after LPS stimulation in vitro (Reference Wlodarska, Luo and Kolde120,Reference Li, Zhang and Wu134) .
In both in vitro experiments and in mice with ulcerative colitis, indole-3-aldehyde has shown the ability to inhibit intestinal damage by targeting inflammatory pathways(Reference Liu, Wang and Xiang136). Additionally, indole-3-aldehyde has been found to upregulate the expression of junction proteins in vitro, as well as in mice with ulcerative colitis(Reference Liu, Wang and Xiang136) and sclerosing cholangitis(Reference D'Onofrio, Renga and Puccetti130). Indole-3-aldehyde has also been observed to restore the expression of the proliferation marker(Reference D'Onofrio, Renga and Puccetti130). Moreover, indole-3-aldehyde has demonstrated the ability to restore antifungal resistance in mice with impaired adaptive immunity, suggesting a potential role in supporting microbial symbiosis(Reference Zelante, Iannitti and Cunha117).
Other indole derivatives have also been implicated in the functioning of the intestinal barrier. For example, indoleacrylic acid has demonstrated anti-inflammatory properties by enhancing the production of IL-10, reducing the secretion of proinflammatory cytokines, and promoting antioxidant responses following LPS stimulation in vitro (Reference Wlodarska, Luo and Kolde120). Indoleacrylic acid has also been associated with enhanced goblet cell function and increased mucus production, possibly through the activation of the AHR, as observed in vitro (Reference Wlodarska, Luo and Kolde120). Meanwhile, tryptamine has shown the ability to decrease inflammatory-induced permeability in vitro (Reference Jennis, Cavanaugh and Leo133). Conversely, indoxyl sulphate, a uremic toxin, has been found to inhibit genes related to tight junctions in vitro (Reference Shimada, Kinoshita and Harada132,Reference Huang, Zhou and Wang137) .
The primary function of kynurenine pathway in the intestine is to maintain immune balance(Reference Lai, Huang and Li138). Treatment with kynurenine can alleviate intestinal inflammation induced by LPS(Reference Gao, Yang and Liu90). Kynurenic acid demonstrates potential anti-inflammatory properties in the gastrointestinal tract by inhibiting inflammatory enzymes(Reference Varga, Erces and Fazekas139) and acting as a ligand for G protein-coupled receptor(Reference Gao, Xu and Liu104). Similarly, quinolinic acid plays a role in immunoregulatory processes within the intestine(Reference Kennedy, Cryan and Dinan54). Furthermore, the expression of IDO1 in the intestine is involved in preserving the integrity of the intestinal barrier and mediating anti-inflammatory effects on the intestinal mucosa through various mechanisms(Reference Schoultz and Keita40,Reference Gao, Xu and Liu104) . For instance, IDO1 has promoted the differentiation of secretory cells of the intestinal epithelial in mice and its increased expression correlated with increased levels of mucin 2 and AHR in both healthy individuals and Crohn's disease patients(Reference Alvarado, Chen and Iticovici140). However, limited evidence exists regarding other roles of the kynurenine pathway in the intestine, emphasising the need for further investigation in this area.
Regarding intestinal barrier functions, serotonin has displayed both protective and detrimental effects on the intestinal system, with outcomes varying based on the methodology employed in different studies. The administration of serotonin has been shown to alleviate LPS-induced intestinal inflammation in vitro (Reference Gao, Yang and Liu90). Neuronal, rather than mucosal, serotonin has promoted intestinal mucosal growth in mice(Reference Gross, Gershon and Margolis141). Contrary, increased levels of mucosal serotonin have been associated with aggravated inflammation(Reference Bischoff, Barbara and Buurman142) and inhibiting the production of mucosal serotonin has shown potential in attenuating inflammation in mice with intestinal inflammation(Reference Margolis, Stevanovic and Li143). Moreover, while serotonin administration led to decreased intestinal permeability in healthy controls, it resulted in decreased expression of the tight junction protein occludin in patients with irritable bowel syndrome(Reference Keszthelyi, Troost and Jonkers144). Conversely, melatonin, derived from serotonin and known for its anti-inflammatory properties, has demonstrated the ability to reduce intestinal permeability(Reference Anderson and Maes145).
Clinical implications
To the best of our knowledge, the relationship between whole grains as a hybrid source of dietary fibre and protein, tryptophan metabolism, intestinal microbiota and intestinal barrier function has not been previously addressed simultaneously. The interplay between these factors is intricate and complex, and disruptions in the delicate balance in the gastrointestinal tract can lead to increased intestinal permeability and the development of associated diseases. Despite the encouraging discoveries in this area of research, the evidence regarding the effects of whole grain consumption on intestine-related activity remains limited. Hence, it is essential to investigate the mechanisms underlying these interactions to enhance both individual and public health outcomes.
It is important to acknowledge the vast individual variability. Defining the precise portion of whole grains and the ideal composition of intestinal microbiota for optimal responses on the intestinal barrier functions is exceptionally challenging due to these inherent individual variations. Moreover, certain gastrointestinal conditions, such as irritable bowel syndrome, can influence how one reacts to certain metabolites or stimuli compared to healthy comparisons. Thus, it is essential that personalised dietary strategies are implemented based on physiological assessments. Despite this complexity, the positive effects of whole grain consumption are inevitable, and even moderate consumption can lead to numerous improvements in both the intestinal environment and overall health.
Intestinal microbiota and intestinal barrier function are crucial factors in mediating the health effects associated with the whole grain consumption. Notably, the effects of the intestinal microbiota and intestinal barrier function extend beyond the colon and can impact other parts of the body. For instance, intestinal microbiota can influence the gut–brain axis. This modulation occurs through the production of various metabolites, particularly tryptophan metabolites, as well as the modulation of the intestinal barrier(Reference Houser and Tansey44,Reference Gao, Mu and Farzi49,Reference Carabotti, Scirocco and Maselli146) . Furthermore, the intestinal microbiota and its metabolic activity are involved in the bidirectional connection between the intestine and the liver, referred to as the gut–liver axis, and are associated with liver diseases through this axis(Reference Bajaj and Khoruts147,Reference Zhao, Gong and Xu148) . These pathways present promising targets for investigating the health impacts of whole grain consumption in the future.
Studies have shed light on the critical role of tryptophan metabolites, particularly indoles, in maintaining the integrity of the intestinal barrier. Further investigations are needed to fully understand the broader physiological significance of these metabolites in the gastrointestinal tract and uncover their potential as a novel approach for supporting both intestinal and overall health. This may require novel methods for determination of concentrations of microbial tryptophan catabolites as well. Moreover, while there is evidence regarding how whole grain consumption can influence growth and metabolic activity of certain bacteria connected with tryptophan metabolism, further investigation is needed to fully comprehend these interactions accurately and potentially uncover previously unknown microorganisms involved in the equation.
Besides the indirect impact of whole grains on tryptophan metabolism through the intestinal microbiota, tryptophan present in whole grains can directly participate in the modulation of metabolism within the colon. Nevertheless, the bioavailability of proteins and other nutrients in the whole grains is restricted due to the presence of fibre and other anti-nutrients. As a result, the potential health benefits derived from these nutrients and whole grains in general are diminished. In addition, despite improvements in assessment techniques, both in vitro and in vivo, numerous challenges persist. In vivo studies are typically expensive and involve invasive procedures, which raise ethical concerns. Because of these ethical limitations, direct access to the human intestinal tract is only allowed under certain circumstances. Furthermore, it is more challenging to control external factors that may introduce confounding effects as well as establishing cause-and-effect conclusions in the habitual environment. Animal studies, in addition to the ethical implications, may produce results that are not extrapolated to human subjects because of the differences in physiology and diet among species. Conversely, in vitro models still possess some limitations in replicating the complex processes of human gastrointestinal environment. In the future, we need more food technological approaches to enhance nutrient bioavailability while concurrently refining accurate methods for assessing bioavailability. However, we need to be aware of the potential impact of increasing the bioavailability of both beneficial and unbeneficial elements found in whole grains.
Discrepancy in defining and assessing whole grain intake further complicates drawing unbiased conclusions about associations. Omics technologies are high-throughput techniques that provide high amounts of data about a specific type of molecules including human and bacterial DNA, RNA, proteins and metabolites(Reference Dai and Shen149). While the use of omics technologies may help to identify many potential biomarkers or biomarker profiles related with whole grain consumption and their impact on health, the application of this information in clinical practice may have some challenges. The challenges relate to sensitivity and specificity of the biomarkers as well as to the vast complexity of the metabolic pathways involved and technical issues such as reproducibility and high false-positive rate of omics technologies(Reference Quezada, Guzmán-Ortiz and Díaz-Sánchez150). Identification of metabolites such as alkylresorcinol, avenacosides and benzoxazinoid-derived phenylacetamide sulphates, and their metabolites, that have been proposed as biomarkers of whole grain consumption(Reference Jawhara, Sørensen and Heitmann151,Reference Landberg, Aman and Hallmans152) , may be linked in the future with health outcomes in human subjects.
To this end, we can conclude that we are just starting to understand the actual complexity of the intestinal factors mediating in part the health impacts of whole grain cereals, whether beneficial or unbeneficial. The investigation of these underlying factors, such as intestinal microbiota and intestinal barrier function, will also produce overall knowledge on diet–microbiota interactions. We need to study how microbial metabolites impact epithelial cells' metabolism as well as related immune functions that contribute to promoting optimal intestinal barrier integrity. In addition, much more research is needed on the faith of plant protein in the intestine and the factors that have an impact on it, both biological and food processing technologies. A highly interesting future is ahead for nutrition and food sciences and related research on intestinal activities, with much needed to understand benefits of whole grains and underlying mechanisms. This will in turn provide knowledge and means to direct the nutritional advice and related development of healthier plant-based foods towards more personalised approaches.
Acknowledgements
The graphical abstract and the figure illustrating tryptophan metabolism were made using BioRender (BioRender – biorender.com).
Financial Support
This project is partly funded from the European Union's Horizon Europe research and innovation programme under grant agreement No 101060247 (MK).
Conflict of Interest
None.
Authorship
V. L. drafted the manuscript and had primary responsibility for final content. C. G.-G. and M. K. participated in and supervised writing and editing the manuscript. All authors read, commented and approved the final manuscript.