- atRA
all-trans-retinoic acid
- eNOS
endothelial NO synthase
- FVPD
fruit- and vegetable-puree-based drinks
CVD is one of the major causes of death in Europe and is responsible for 4·3×106 deaths each year across the continent(Reference Allender, Scarborough and Peto1). One of the emerging risk factors for CVD is dysfunction of the endothelium(Reference Bonetti, Lerman and Lerman2, Reference Zeiher3), which is characterised by a reduction in the bioavailability of vasodilators, predominantly NO, endothelium-derived hyperpolarizing factor and prostacyclin, and an increase in endothelium-derived vasoconstrictors, e.g. thromboxane A2, PGH2 and endothelin 1(Reference Bonetti, Lerman and Lerman2). Endothelial dysfunction can be assessed by measuring enhanced and maintained endothelial activation and impaired endothelium-dependent vasodilation(Reference Brown and Hu4).
Endothelial activation is determined by an increase in the plasma concentrations of soluble cell adhesion molecules, such as intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin and P-selectin, because these molecules are shed and released into the plasma from the activated endothelium. Elevated concentrations of these molecules have been found in patients with all types of CVD and those patients who have not developed CVD but display coronary risk factors such as smoking, hypertension, hypercholesterolaemia and diabetes mellitus(Reference Lopez-Garcia, Schulze and Fung5). Consequently, endothelial dysfunction is thought to represent a key early stage in the development of atherosclerosis.
Impaired endothelium-dependent vasodilation in patients with CVD was first reported in 1986(Reference Ludmer, Selwyn and Shook6). When acetylcholine was injected into the coronary arteries of patients vasoconstriction was found instead of the expected relaxation and vasodilation response. Established cardiac risk factors such as age, gender, hypertension, hyperlipidaemia, diabetes mellitus and smoking, as well as novel risk factors such as inflammation and hyperhomocysteinaemia, have all been associated with abnormal vasorelaxation(Reference Kuvin and Karas7). There are now substantial data relating impaired endothelial vasomotor function to CHD(Reference Sattar, Petrie and Jaap8). Assessment of coronary artery endothelial function is commonly achieved by intracoronary infusion of acetylcholine, as it causes release of NO and consequently coronary artery dilation(Reference Kuvin and Karas7). Patients with risk factors for atherosclerosis exhibit a diminished vasodilatory response to acetylcholine or paradoxical vasoconstriction(Reference Ludmer, Selwyn and Shook6). Non-invasive methods of assessing endothelial-dependent vasodilation have now been developed(Reference Sattar and Ferns9). The most frequently used are flow-mediated dilation and laser Doppler imaging with iontophoresis.
The flow-mediated dilation technique uses ultrasonic assessment of brachial artery flow during hyperaemia(Reference Kuvin and Karas7). Essentially, forearm or hand ischaemia is induced when the arterial blood supply is interrupted with a cuff inflated to suprasystolic pressure. When the tourniquet is released dilation of the distal microvasculature induces reactive hyperaemia. NO is released locally as a result of the shear stress and changes in hydrostatic pressure during the hyperaemia. The extent of endothelial function is then elucidated from the magnitude of the change in vessel diameter from baseline to the peak observed during reactive hyperaemia.
It has recently been determined that endothelial dysfunction also occurs in the peripheral circulation and the extent of dysfunction is proportional to that occurring in the coronary arteries(Reference Anderson, Uehata and Gerhard10). Iontophoresis is a technique of investigating the vasodilation of the peripheral microvasculature by delivering vasodilator agents across the skin under the influence of an applied electrical field. Iontophoresis coupled with laser Doppler flowmetry has been used as a non-invasive technique for measuring microvascular perfusion at a single point(Reference Ferrell, Ramsay and Brooks11). However, an emerging method combining iontophoresis with laser Doppler imaging enables the measurement of perfusion across many points and an average measure of perfusion can then be calculated for a given area(Reference Ferrell, Ramsay and Brooks11). Acetylcholine is administered at the anode and tests endothelial function via its vasodilator action of binding to muscarinic receptors on endothelial cells and subsequently generating NO; consequently, acetylcholine is known as an ‘endothelium-dependent’ vasodilator. As vasodilation is ultimately mediated by the action of NO on vascular smooth muscle a NO donor, sodium nitroprusside, is administered at the cathode. This compound is used as an ‘endothelium-independent’ vasodilator and acts as a control to test the integrity of vascular smooth muscle. Examination of the dilation response of skin microvasculature to acetylcholine and sodium nitroprusside has shown that the local vasodilatory response is reduced in patients with peripheral arterial occlusive disease compared with age- and gender-matched healthy controls(Reference Jagren, Gazelius and Ihrman-Sandal12).
The effects of phytochemicals from fruits and vegetables on endothelial dysfunction
There are several components of fruit and vegetables that have been shown to exert favourable effects on vascular endothelial function, either by improving endothelium-dependent vasodilation or by decreasing endothelial activation. These components include: folic acid(Reference Wilmink, Stroes and Erkelens13); the antioxidant vitamins C (ascorbic acid) and E (α-tocopherol)(Reference Devaraj, Li and Jialal14, Reference Chambers, McGregor and Jean-Marie15); polyphenols(Reference Andriambeloson, Magnier and Haan-Archipoff16, Reference Taubert, Berkels and Klaus17); carotenoids(Reference Suganuma and Inakuma18); fibre(Reference Brock, Davis and Irving19).
The effects of folic acid on endothelial dysfunction
Research has shown that folic acid can have a beneficial effect on the vascular endothelium by reducing plasma homocysteine levels(Reference Wilmink, Stroes and Erkelens13). This effect is a result of the role of folic acid during the methylation of homocysteine to methionine(Reference Malinow, Bostom and Krauss20). Homocysteine has several detrimental effects on the vascular endothelium, which include: increasing platelet aggregation and thrombosis(Reference Brown and Hu4); increasing oxidative stress by increasing superoxide production(Reference McDowell and Lang21); down regulating NO production(Reference Pruefer, Scalia and Lefer22); increasing vascular smooth muscle proliferation(Reference Tsai, Perrella and Yoshizumi23); increasing leucocyte–endothelium interactions(Reference Dudman, Temple and Guo24).
There have been several clinical studies that have investigated the effects of folic acid supplementation on endothelial function. It has been reported that when subjects who are mildly hyperhomocysteinaemic are supplemented with folic acid (5 mg/d) and pyridoxine (250 mg/d) over a 12-week period a decrease is found in both plasma von Willebrand factor and thrombomodulin(Reference Van den Berg, Boers and Franken25). Another study in which subjects received folic acid (5 mg/d) chose to investigate forty subjects with familial hypercholesterolaemia(Reference Verhaar, Wever and Kastelein26). After 4 weeks it was found that folic acid supplementation restores endothelium-dependent vasodilation, as assessed by serotonin-induced forearm blood flow measurement. In a further intervention study eighteen subjects with hyperhomocysteinaemia were given 5 mg folic acid daily during a 6-week intervention period(Reference Bellamy, McDowell and Ramsey27). Flow-mediated vasodilation of the brachial artery was measured and it was found that supplementation improves endothelium-dependent vasodilation.
Two intervention studies of folic acid supplementation have been reported that used healthy subjects(Reference Wilmink, Stroes and Erkelens13, Reference Woo, Chook and Lolin28). Both studies used 10 mg folic acid/d or placebo and measured the outcome by flow-mediated vasodilation of the brachial artery. One study comprising seventeen subjects with an intervention period of 8 weeks has shown an improvement in endothelium-dependent vasodilation(Reference Woo, Chook and Lolin28). The other study of twenty subjects who received the supplement for a 2-week period carried out vasomotion measurements on each subject before and 4 h after an oral fat load (50 g, in the form of whipped cream)(Reference Wilmink, Stroes and Erkelens13). It was found that folic acid supplementation prevents vasodilation impairment after a fat load.
These supplementation studies suggest that folic acid has a beneficial effect on endothelial function, as measured by haemostatic markers or flow-mediated vasodilation. Although it is suggested that the observations are a result of reduced homocysteine concentrations, other mechanisms may also contribute to the effects.
The effects of vitamins C and E on endothelial dysfunction
Endothelial function may be modulated by antioxidant vitamins such as vitamin C and vitamin E. Animal experiments have shown that one form of vitamin E, α-tocopherol, preserves NO-mediated vascular relaxation in subjects with hypercholesterolaemia(Reference Andersson, Matz and Ferns29, Reference Stewartlee, Forster and Nouroozzadeh30). In vitro studies with antioxidant vitamins have shown that vitamin E reduces cell adhesion molecule expression(Reference Wu, Koga and Martin31) and monocyte adhesion to the endothelium(Reference Faruqi, Delamotte and Dicorleto32) and vitamin C leads to improvement in endothelium-dependent vasodilation(Reference Fontana, Mcneill and Ritter33).
A number of clinical studies have investigated the effects of antioxidant supplementation, in the form of vitamins C or E, on markers of endothelial activation or endothelium-dependent vasodilation. These studies have found a decrease in monocyte adhesion with both vitamin C(Reference Weber, Erl and Weber34) and vitamin E(Reference Devaraj, Li and Jialal14), and plasma P-selectin is reduced in subjects with hypercholesterolaemia following α-tocopherol supplementation(Reference Davi, Romano and Mezzetti35). It has been reported that infusion with vitamin C leads to an increase in forearm blood flow of 1·4 ml/100 ml per min in subjects after a 6 h hyperglycaemic clamp(Reference Beckman, Goldfine and Gordon36). Vitamin C supplementation has been shown to improve endothelium-dependent vasodilation, measured by flow-mediated dilation, from 4·2 (se 0·7) % to 9·1 (se 1·3) % in subjects with chronic heart failure(Reference Hornig, Arakawa and Kohler37) and from 6·6 (sd 3·5) % to 10·1 (sd 5·2) % in subjects with coronary artery disease(Reference Gokce, Keaney and Frei38). In healthy subjects in whom hyperhomocysteinaemia has been induced with an oral methionine load the reduction in flow-mediated dilation can be ameliorated by pre-treatment with vitamin C supplementation of 1000 mg/d for 1 week(Reference Chambers, McGregor and Jean-Marie15). In smokers supplemented with vitamin E (545 mg all-racemic α-tocopherol/d) there is an improvement in flow-mediated vasodilation of 5·8 (sd 3·2) % for the supplemented group v. 2·7 (sd 2·8) % for the placebo group(Reference Neunteufl, Priglinger and Heher39).
Dilation of blood vessels occurs when endothelial cells generate NO(Reference Brown and Hu4). This molecule can react with superoxide generated by endothelial cells during cytoplasmic and mitochondrial metabolism to form peroxynitrite, which is destructive to cells(Reference Ischiropoulos40). Antioxidants could therefore decrease this consumption of NO and render it bioavailable for vasodilation(Reference Bendich, Machlin and Scandurra41). However, it has been found that high physiological concentrations of vitamin C (>1 mM) are needed to effectively scavenge superoxide and enhance endothelium-dependent vasodilation(Reference Jackson, Xu and Vita42). Vitamin C could augment delivery of NO from the plasma to the vascular cells(Reference May43). NO can be transported as S-nitrosothiols on albumin or free cysteine(Reference Keaney, Simon and Stamler44) and vitamin C can release NO from S-nitrosothiols and S-nitrosoalbumin(Reference Scorza, Pietraforte and Minetti45). However, as most of the free NO is likely to be scavenged by Hb and erythrocytes(Reference Jia, Bonaventura and Bonaventura46, Reference Patel, Hogg and Spencer47) further investigations are required to determine whether sufficient NO can be released by this mechanism to promote vasodilation. Another mechanism whereby vitamin C could increase vasodilation is by regulating endothelial NO synthase (eNOS) by increasing the affinity of eNOS for tetrahydrobiopterin(Reference Heller, Munscher-Paulig and Grabner48). This outcome could be achieved by preserving crucial thiol groups on eNOS, which are needed in order to bind tetrahydrobiopterin(Reference Hofmann and Schmidt49), or by reducing the redox cycling of tetrahydrobiopterin by decreasing cellular superoxide or peroxynitrite(Reference Kojima, Ona and Iizuka50).
Overall, the studies generally show a supportive role for antioxidant vitamins C and E in preserving endothelium-dependent vasodilation. This effect is particularly evident in subjects with cardiovascular risk factors such as hyperlipidaemia or in patients with diabetes or established CVD.
The effects of carotenoids on endothelial dysfunction
The addition of tomato to the diet has been reported to have a protective effect on acetylcholine-induced vasorelaxation in hypercholesterolaemic mice fed on an atherogenic diet(Reference Suganuma and Inakuma18). Mice fed a diet high in saturated fat and cholesterol for 4 months were found to have an increased plasma lipid peroxide level and a decreased vasorelaxing activity in the aorta induced by acetylcholine compared with mice on a commercial diet. However, when 20% (w/w) powdered tomato was added to the atherogenic diet a lesser increase in the plasma lipid peroxide level was found and acetylcholine-induced vasorelaxation was maintained at the same level as that for normal mice. Although it was concluded that tomato has a preventive effect on atherosclerosis by protecting plasma lipids from oxidation, the nature of the protective factor in the tomato could not be determined. However, it was noted that tomatoes contain a relatively large amount of the carotenoid lycopene and that the tomato powder used in the study contained 1·6 mg lycopene/g. Based on a report that indicates that lycopene supplementation inhibits singlet-oxygen-mediated oxidation of human plasma LDL(Reference Oshima, Ojima and Sakamoto51) and the finding that the uptake of tomato reduces the increase in plasma lipid peroxide in hypercholesterolaemic mice, it was suggested that lycopene might act as an antioxidant in plasma(Reference Suganuma and Inakuma18). However, plasma lycopene was not measured in the mouse study and it was noted that carotenoids are thought to be less absorbed by rodents than by human subjects. Thus, it was concluded that the findings strongly suggest that dietary ingestion of tomato inhibits the oxidative modification of serum lipids and subsequently reduces endothelial dysfunction(Reference Suganuma and Inakuma18).
The evidence in support of a preventative function of lycopene in CVD primarily originates from epidemiological observations in normal and at-risk populations. The most powerful population-based evidence has been derived from a European multicentre case–control study(Reference Kohlmeier, Kark and Gomezgracia52). This study investigated the relationship between acute myocardial infarction and adipose tissue antioxidant status. Levels of α- and β-carotenes, lycopene and α-tocopherol were measured in adipose tissue collected shortly after the infarction. However, after adjustment for age, BMI, socio-economic status, smoking, hypertension and family history of the disease, only lycopene levels were found to be protective. The results show a dose–response relationship between each quintile of adipose tissue lycopene content and the risk of myocardial infarction.
Although the European multicentre case–control study observed no protective effect of β-carotene or α-tocopherol on myocardial infarction(Reference Kohlmeier, Kark and Gomezgracia52), other research has shown that these lipophilic antioxidants preserve endothelium-dependent relaxation in cholesterol-fed rabbits(Reference Keaney, Gaziano and Xu53). Male rabbits were divided into four groups; one group was fed a commercial diet, another group received the commercial diet with 1% (w/w) cholesterol added and the final two groups received the 1% (w/w) cholesterol diet with either β-carotene (0·6 g/kg chow) or α-tocopherol (1000 mg α-tocopheryl acetate/kg chow). After 28 d on the diets thoracic aortas were collected and analysed for vascular function and tissue antioxidant levels. Acetylcholine-induced vasorelaxation was found to be significantly impaired in vessels from the cholesterol group (P<0·001), whereas vessels from the animals supplemented with β-carotene or α-tocopherol displayed normal arterial relaxation. Preservation of vasorelaxation was found to be associated with vascular incorporation of β-carotene and α-tocopherol, but unrelated to plasma lipoprotein levels, smooth muscle cell function or the extent of atherosclerosis. Increased LDL resistance to ex vivo Cu-mediated oxidation was only found in the α-tocopherol group. As dysfunction in endothelium-dependent vasorelaxation is associated with risk of CVD(Reference Anderson, Uehata and Gerhard10), this research may suggest that the benefit of diets containing carotenoids on this risk factor may be through vascular tissue antioxidant content and not associated with the resistance of LDL to ex vivo oxidation.
The pro-vitamin A activity of β-carotene is well known(Reference Dimascio, Murphy and Sies54) and vitamin A derivatives, particularly all-trans-retinoic acid (atRA), have been shown to inhibit cellular proliferation and promote cellular differentiation(Reference Achan, Tran and Arrigoni55). In the latter study it was found that atRA increases NO synthesis by endothelial cells. Murine endothelioma cells treated with or without atRA were found to show a maximum increase in nitrite production 24 h after stimulation with atRA. eNOS mRNA expression was not found to increase after treatment with atRA and there was no detectable eNOS or inducible NO synthase mRNA in the cells. The effect of atRA on the induction of the dimethylarginine dimethylaminohydrolase enzymes that regulate asymmetric dimethylarginine metabolism was also investigated. Dimethylarginine is an endogenous competitive inhibitor of NOS and dimethylarginine dimethylaminohydrolase II has been shown to be highly expressed in cardiovascular tissues and to have a distribution similar to eNOS(Reference Leiper, Maria and Chubb56). It was found that dimethylarginine dimethylaminohydrolase II expression is induced by atRA, with the maximum increase occurring 12 h after stimulation(Reference Achan, Tran and Arrigoni55). This effect was also observed in primary porcine aortic endothelial cells and the human cell lines SGHEC-7 (SV40 transfected human umbilical vein endothelial cells) and ECV304. As raised levels of dimethylarginine have been shown to be associated with endothelial dysfunction and atherosclerosis(Reference Miyazaki, Matsuoka and Cooke57, Reference Boger, Bode-Boger and Szuba58) it was suggested that induction of dimethylarginine dimethylaminohydrolase II by atRA may lower dimethylarginine levels and consequently restore NO production.
These studies support the epidemiological findings that carotenoid-containing foods may be beneficial in reducing the risk of CVD(Reference Cherubini, Vigna and Zuliani59). However, they suggest that their mode of action may not be entirely a result of their antioxidant properties.
The effects of polyphenols on endothelial dysfunction
In 1993 it was reported that grape juice and grape-skin extracts cause endothelium-dependent vasorelaxation(Reference Fitzpatrick, Hirschfield and Coffey60). The study focused on the effect of certain wines, grape juices and grape-skin extracts on precontracted smooth muscle of rat aortic rings. Relaxation was found in intact aortic rings, but no effect was observed on aortas in which the endothelium had been removed. The polyphenolic compounds quercetin and tannic acid were found to produce endothelium-dependent relaxation. The grape extracts were found to increase cGMP levels in the intact vascular tissue. Both relaxation and the increase in cGMP were reversed by NG-monomethyl-l-arginine and NG-nitro-l-arginine, which are competitive inhibitors of NO synthesis. These findings suggest that grape-product-induced vasorelaxation is mediated by the NO–cGMP pathway. To further investigate the effect of various plant extracts on endothelium-dependent vasorelaxation aqueous extracts of numerous fruits, vegetables, nuts, herbs, spices and teas were studied(Reference Fitzpatrick, Hirschfield and Ricci61). It was found that many of, but not all, these extracts exhibit endothelium-dependent relaxation that is again reversed in the presence of a NO synthase inhibitor.
Following on from the initial work with grape products performed on rat aorta(Reference Fitzpatrick, Hirschfield and Coffey60, Reference Fitzpatrick, Hirschfield and Ricci61), other studies on various isolated animal and human blood vessels have reached similar conclusions(Reference Stoclet, Chataigneau and Ndiaye62). These studies used a variety of plant polyphenol sources such as cocoa, tea, hawthorn (Crataegus monogyna and Crataegus oxyacantha fruit), honey and pine (Pinus maritima) bark. All these studies have found that plant polyphenols produce endothelium-dependent vasorelaxation that is associated with increased cGMP formation and reduced by NO synthase inhibitors.
Structure–activity (endothelial NO release and relaxation of isolated blood vessels) relationships for different classes of polyphenols have been examined(Reference Andriambeloson, Magnier and Haan-Archipoff16, Reference Taubert, Berkels and Klaus17). The most active fractions in red wine were found to be flavan-3-ol-enriched oligomeric condensed tannins, particularly dimers and trimers, and the most active monomer was the anthocyanin delphinidin(Reference Andriambeloson, Magnier and Haan-Archipoff16). Surprisingly, structurally-similar anthocyanins, such as malvidin and cyanidin, were not found to be active. However, substitution of the flavan moiety with free hydroxyl residues at precise locations has been found to be important to induce endothelial NO release(Reference Taubert, Berkels and Klaus17).
The mechanisms of eNOS activation in response to circulating hormones, local autacoids and substances released by platelets, by the coagulation cascade and by the autonomic nervous system involve intracellular Ca2+ (Reference Dimmeler, Fleming and Fisslthaler63). This role of Ca2+ is supported by the relaxation of rat aorta(Reference Andriambeloson, Stoclet and Andriantsitohaina64) and the increase in Ca2+ concentration in bovine aortic endothelial cells(Reference Stoclet, Kleschyov and Andriambeloson65) caused by a polyphenolic extract from alcohol-free red wine and by delphinidin, which are both Ca2+ dependent effects. Furthermore, wine polyphenols are unable to increase Ca2+ concentration in smooth muscle cells, indicating the selectivity of this effect(Reference Stoclet, Kleschyov and Andriambeloson65).
Long-term incubation of endothelial cells with red wine increases eNOS expression(Reference Wallerath, Deckert and Ternes66, Reference Wallerath, Poleo and Li67). This effect is only achieved, however, with large volumes of red wine (1% (v/v) in culture medium for 10 d, 3% (v/v) for 24 h and 10% (v/v) for 12 h)(Reference Wallerath, Poleo and Li67). Resveratrol, also a polyphenolic component of red wine, stimulates eNOS expression in the concentration range of 10–100 μM, as well as increasing the activity of the eNOS promoter and stabilising eNOS mRNA(Reference Wallerath, Deckert and Ternes66).
In a study of the incorporation of elderberry (fruit of elder; Sambucus nigra) anthocyanins by bovine and human aortic endothelial cells treated with an elderberry extract (1 mg/ml) and incubated for 1, 2, 4, 6, 8, 16 and 24 h, maximum incorporation was found after 4 h(Reference Youdim, Martin and Joseph68). By separation of the cell membranes and cytosol it was demonstrated that the anthocyanins were incorporated into the plasma membrane as well as penetrating into the cell cytosol. Uptake into both regions was found to be structure dependent, with monoglycoside concentrations higher than those of the diglucosides. The incorporation of elderberry anthocyanins was found to be protective against H2O2, 2,2′-azobis(2-amidinopropane) dihydrochloride and FeSO4–ascorbic acid.
A protective effect of blackberry (Rubus fruticosus) anthocyanins, notably cyanidin-3-O-glucoside, on endothelial cells has also been reported(Reference Serraino, Dugo and Dugo69). In this study the effect of peroxynitrite on human umbilical vein endothelial cells incubated with differing concentrations of blackberry juice was investigated. It was found that blackberry juice reduces the peroxynitrite-induced suppression of mitochondrial respiration, DNA damage and poly (ADP-ribose) synthetase activation in human umbilical vein endothelial cells. It was also reported that vascular rings exposed to peroxynitrite demonstrate reduced endothelium-dependent relaxant responses to acetylcholine, which is improved by the blackberry juice.
In addition to research with endothelial cells, polyphenols have also been incubated with platelets(Reference Freedman, Parker and Li70). Incubation of platelets with diluted purple-grape juice leads to inhibition of aggregation, enhanced release of platelet-derived NO and decreased superoxide production(Reference Freedman, Parker and Li70). In order to investigate these effects in vivo, an intervention trial was conducted in which twenty healthy subjects consumed 7 ml purple-grape juice/kg daily for 14 d. Following the intervention platelet aggregation was found to be inhibited, platelet-derived NO production increased from 3·5 pmol/108 platelets to 6·0 pmol/108 platelets and superoxide release decreased from 29·5 arbitrary units to 19·2 arbitrary units. It was also reported that α-tocopherol levels significantly increase and plasma protein-independent antioxidant activity increases by 50%.
Other intervention trials have investigated the effect of a range of polyphenols on endothelial dysfunction(Reference Whelan, Sutherland and Mccormick71, Reference Duffy, Keaney and Holbrook72). In a study of the effects of white and red wines on the endothelial function of fourteen subjects with coronary artery disease 4 ml wine/kg was administered alongside a controlled meal low in antioxidants(Reference Whelan, Sutherland and Mccormick71). Measurement of flow-mediated dilation of the brachial artery indicated an improvement in dilation after 360 min, although no difference was found between the two types of wine. Consumption of an isoenergetic cordial was not found to improve dilation. No difference in plasma polyphenols was observed in blood samples taken at baseline, 60 min and 360 min after consumption of either type of wine.
In another study of patients with coronary artery disease fifty patients with proven coronary artery disease underwent an intervention trial of black tea consumption(Reference Duffy, Keaney and Holbrook72). Short-term effects were measured by examining the subjects 2 h after consumption of 450 ml tea or water and long-term effects were measured after consumption of 900 ml tea or water daily for 4 weeks. Measurements of flow-mediated vasodilation of the brachial artery showed improvement with both the short- and long-term tea consumption, whereas water was found to have no effect. Administration of an equivalent oral dose of caffeine was not found to have any short-term effect on flow-mediated vasodilation. Plasma concentrations of flavonoids were shown to increase after both the short- and long-term tea consumption. Although tea contains antioxidant flavonoids, studies have shown that tea consumption has no effect on plasma antioxidant capacity, plasma concentrations of F2-isoprostanes, markers of systemic lipid peroxidation or 8-hydroxydeoxyguanosine(Reference Vita73).
Many fruits and vegetables contain polyphenolic flavonoid compounds(Reference Tomas-Barberen and Clifford74, Reference Hollman and Arts75), some of which have been shown to increase the activity of the eNOS enzyme(Reference Schroeter, Heiss and Balzer76). eNOS is responsible for the generation of the vasodilator NO from l-arginine in the vascular endothelium(Reference Moncada and Higgs77). A common single-nucleotide polymorphism (G298T) occurs in the eNOS gene that modifies its coding sequence, replacing a glutamate residue at position 298 with an aspartate residue, and this polymorphism has been linked to increased risk of cardiovascular events(Reference Hingorani, Liang and Fatibene78–Reference Hibi, Ishigami and Tamura82).
Two studies have been conducted to investigate the effects of chronic and acute consumption of commercially-available fruit and vegetable puree-based drinks (FVPD) on vasodilation, phytochemical bioavailability and antioxidant status in healthy individuals aged between 30 and 70 years (TW George, C Niwat, S Waroonphan, MH Gordon and JA Lovegrove, unpublished results; TW George, E Paterson, S Waroonphan, MH Gordon and JA Lovegrove, unpublished results). FVPD were chosen to provide a convenient source of fruit and vegetable nutrients and phytochemicals such as vitamin C and flavonoids. FVPD enabled all the participants to receive a standardised product throughout the duration of the studies, which would have been difficult to achieve with fresh produce. The participants of both studies were retrospectively genotyped for the G298T polymorphism and their endothelium-dependent vasodilation responses re-analysed in relation to genotype.
Materials and methods
Study participants
Thirty-nine participants completed the chronic study (twenty-four females and fifteen males, mean age (years) 45 (sd 9) and 44 (sd 11) respectively) and twenty-four completed the acute study (twenty males and four females, mean age (years) 46 (sd 11) and 49 (sd 4) respectively). The participants in both studies were recruited if they met the criteria: no known liver disease, diabetes mellitus or a diagnosed mycocardial infarction; no gall bladder problems or abnormalities of fat metabolism; no subjects on weight-reducing diets or taking dietary supplements; no vigorous exercise or excess consumption of alcohol; BMI <30 kg/m2; blood pressure <150/90 mmHg and Hb >125 g/l. Basic anthropometric measurements were recorded for all study participants and fasting blood was taken and analysed for liver function status, lipid levels, fasting glucose and a measure of potential alcohol abuse. The studies were approved by the University of Reading Research Ethics Committee and each participant gave informed consent before participating.
Study designs
The chronic study was a single-blind randomised controlled cross-over dietary study. After a 1-week run-in period with control drink (lemon barley water; Robinsons Ltd, Chelmsford, Essex, UK), the participants consumed the test intervention of two FVPD (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany) per d or a control drink of diluted fruit-flavoured cordial for a 6-week period (for details of drinks, see later). After 8 weeks the participants were asked to repeat the intervention but consuming the other drink. Participants completed two 5 d diet records (four weekdays and one weekend day) during each cross-over arm of the study (four diaries in total for each subject). Fasting plasma and urine samples were collected at baseline and at the end of the intervention period in each arm of the study. In addition, a real-time measure of vascular tone using laser Doppler iontophoresis before and after the intervention period was performed on a subset of the participants (n 19) in one arm of the study in a parallel study design.
The acute study was a single-blind controlled cross-over dietary study. The participants consumed a low-flavonoid diet for the 5 d preceding the study day. On the study day a flexible cannula was inserted into the forearm and blood samples were taken at baseline and at twelve additional time points after consumption of the relevant drink (eight samples 30 min apart followed by four samples 1 h apart). Urine was collected before the drink (FVPD or sugar-matched control) was consumed and then at two-hourly intervals for the 8 h of the study day. Laser Doppler iontophoresis measurements were recorded at baseline and at five 90 min intervals following drink consumption. The whole procedure was repeated with the other intervention drink after a 4-week washout period.
Intervention drinks
For the chronic study participants were asked to consume two bottles (2×100 ml) of FVPD daily. The FVPD were fruit and vegetable preparations made from purees and concentrated juices, which contained the equivalent of 200 g fruit and vegetables per bottle. Participants were asked to consume daily one bottle of apple, carrot and strawberry and one bottle of orange, banana and carrot drink. The control drink was either orange or lemon barley water (50 ml diluted to 400 ml with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK) daily). The nutrient composition of the FVPD and the control drinks is shown in Tables 1 and 2 respectively.
Table 1. Nutrient composition (/100 ml) of fruit- and vegetable-puree-based drinks
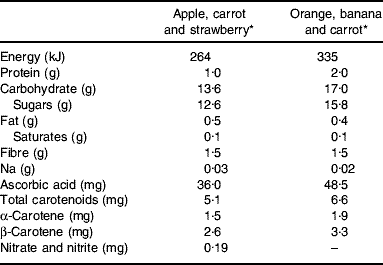
* Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany.
Table 2. Nutrient composition (/50 ml) of control drinks

* Robinsons Ltd, Chelmsford, Essex, UK.
† 50 ml fruit-flavoured cordial (lemon barley water; Robinsons Ltd), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK).
For the acute study participants were asked to consume 400 ml FVPD (apple, carrot and strawberry) or 50 ml fruit-flavoured cordial (lemon barley water), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd).
Blood sample collection
Blood samples were collected into separate vacutainer tubes containing citrate, EDTA and heparin anticoagulants, which were immediately wrapped in foil and kept on ice for transport to the laboratory. Each subject's blood samples were centrifuged at 4°C at 3000 rpm immediately after collection, plasma and buffy coat were retained, aliquots of plasma were put into separate cryogenic vials for storage and all samples were frozen for future analysis. The objective of this approach was to minimise oxidative changes within the plasma sample before storage at −80°C. Analyses were not carried out until the intervention study had been completed and then all samples for each subject were analysed within one batch to reduce inter-batch variation.
Laser Doppler imaging with iontophoresis
Vascular reactivity was recorded for a subgroup of nineteen of the thirty-nine participants in the chronic study and for all twenty-four participants in the acute study. Measurements were taken with each subject in a supine position in a quiet room maintained at an ambient temperature of 22±1°C. Two ION6 chambers (Moor Instruments Ltd, Axminster, Devon, UK) were placed on the forearm and connected to a MIC2 iontophoresis controller (Moor Instruments Ltd). Acetylcholine chloride (Sigma Aldrich, Poole, Dorset, UK; 2·5 ml; 1% (w/v) in 0·5% (w/v) NaCl solution) was placed in the anodal chamber and sodium nitroprusside (Sigma Aldrich; 2·5 ml; 1% (w/v) in 0·5% (w/v) NaCl solution) was placed in the cathodal chamber. Current delivery was controlled by laser Doppler imager Windows software (Moor Instruments Ltd). Measurement of skin perfusion was carried out using a laser Doppler imager (moorLDI2; Moor Instruments Ltd). Repeat scans were taken with increasing current, to give a total charge of 8 mC. The area under the flux v. time curve represented the microvascular response.
Plasma lipid profiles and plasma glucose
Analyses of plasma TAG, total cholesterol, HDL-cholesterol, NEFA and glucose hexokinase were performed using an Instrument Laboratory ILAB 600 autoanalyzer and standard kits (Instrumental Laboratories Ltd, Warrington, Ches., UK). Appropriate sero-normal, low- and high-quality control standards (Instrument Laboratories Ltd) were included in all batches.
Plasma insulin
Insulin was assessed by ELISA (Dako Cytomation, Ely, Cambs., UK) with in-house pooled plasma controls in each batch.
Plasma ascorbic acid and uric acid
Plasma samples were treated with an equal volume of 10% (v/v) metaphosphoric acid, centrifuged at 12 000 rpm for 10 min and the supernatant fraction stored at −80°C before analysis for ascorbic acid by HPLC with UV detection(Reference Liau, Lee and New83) and simultaneous determination of uric acid(Reference Ross84).
Plasma carotenoids and tocopherols
Plasma carotenoids and tocopherols were measured by the method of Thurnam et al.(Reference Thurnham, Smith and Flora85) using a Hewlett Packard 1050 HPLC system with a Nucleosil 100-5C18 column (250 mm×46 mm; Hichrom Ltd, Reading, Berks., UK) at a flow rate of 1·5 ml/min and the detector set to monitor at 292 nm for α- and γ-tocopherol, 450 nm for lutein, β-cryptoxanthin, α-carotene and β-carotene and 472 nm for lycopene.
Total nitrite and nitrate
Total nitrate and nitrite was measured using an ELISA kit (Active Motif, Rixensart, Belgium) based on the Greiss reaction(Reference Green, Wagner and Glogowski86) for plasma, FVPD and the control drink.
Analysis of Glu298Asp polymorphism
DNA was isolated from the buffy coat layer of 10 ml blood collected in an EDTA vacutainer using the Qiagen DNA Blood Mini Kit (Qiagen Ltd, Crawley, West Sussex, UK). Allelic discrimination of the eNOS gene variants was conducted using TaqMan PCR technology (7300 Instrument; Applied Biosystems, Warrington, Ches., UK) and Assay-on-Demand SNP genotyping assays (Applied Biosystems).
Dietary analysis
The diet diaries were analysed using Diet Cruncher nutritional analysis software (Way Down South Software, Dunedin, New Zealand) in combination with an electronic version of the McCance and Widdowson 6th edition food composition database (Food Standards Agency, London, UK).
Statistical analyses
All statistical analyses were performed using SPSS 13.0 for Microsoft Windows (SPSS Inc., Chicago, IL, USA). The data were assessed for normality using the Shapiro–Wilk test, as the number of subjects was less than fifty. Those data that were not normally distributed were log transformed and reassessed. A repeated measure ANOVA was used to test for differences between treatment groups, with Bonferroni correction to reduce the likelihood of chance findings from multiple comparisons. A value of P⩽0·05 was used to define significance and a 95% CI. The data are presented as means with their standard errors, unless otherwise stated.
Results
Chronic FVPD consumption
Table 3 summarises the major findings from the chronic FVPD consumption study. There was a significant increase in dietary carotenoids and ascorbic acid following FVPD consumption (P=0·001 and P=0·003 respectively). There was an increase in plasma carotenoids and ascorbic acid, which was significant for plasma carotenoids (P=0·001). There was no significant effect of FVPD on measures of oxidative stress, oxidative stability, lipid profiles, plasma homocysteine, von Willebrand factor, C-reactive protein or on the susceptibility of LDL particles to oxidation (data not shown).
Table 3. Summary of dietary and plasma carotenoid and ascorbic acid concentrations before and after 6 weeks of chronic consumption of fruit- and vegetable-puree-based drinks (FVPD) or control (n 39)Footnote *

* The FVDP drink was one 100 ml bottle of apple, carrot and strawberry and one 100 ml bottle of orange, banana and carrot (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany) daily and the control drink was 50 ml lemon barley water (Robinsons Ltd, Chelmsford, Essex, UK) diluted to 400 ml with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK) daily. For details of studies, see text.
† Change over time in the FVPD group.
Acute FVPD consumption
The participants exhibited a lower glucose and insulin peak concentration after consumption of the FVPD compared with the sugar-matched control (Figs. 1 and 2 respectively). There was a significant time×treatment effect on plasma glucose and insulin (P=0·019 and P=0·003 respectively). There was a significant time×treatment effect of FVPD consumption on plasma ascorbic acid and total plasma nitrate and nitrite (P=0·002 (Fig. 3) and P=0·001 (Fig. 4) respectively). There was a significant increase in plasma ascorbic acid from 60 min until the end of the study day (Fig. 3). There was an increase in total plasma nitrate and nitrite at all time points following FVPD consumption (Fig. 4).
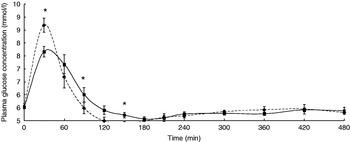
Fig. 1. Plasma glucose concentration (mmol/l; n 24) following acute consumption of fruit- and vegetable-puree-based drinks (400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany); ▪––▪) or control (50 ml fruit-flavoured cordial (lemon barley water; Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); ◆- - -◆). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·019). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
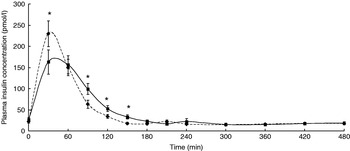
Fig. 2. Plasma insulin concentration (pmol/l; n 24) following acute consumption of fruit- and vegetable-puree-based drinks (400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany); ▪––▪) or control (50 ml fruit-flavoured cordial (lemon barley water; Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); ◆- - -◆). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·003). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
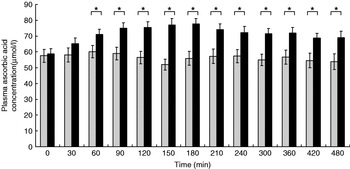
Fig. 3. Plasma ascorbic acid concentration (μmol/l; n 24) following acute consumption of fruit- and vegetable-puree-based drinks (400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany); ▪) or control (50 ml fruit-flavoured cordial (lemon barley water; Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); ![]() ). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·002). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·002). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
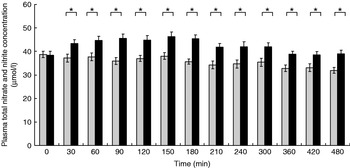
Fig. 4. Total plasma nitrate and nitrite concentration (μmol/l; n 24) following acute consumption of fruit- and vegetable-puree-based drinks (400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany); ▪) or control (50 ml fruit-flavoured cordial (lemon barley water; Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); ![]() ). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·001). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
). Values are means with their standard errors represented by vertical bars. The time×treatment effect was significant (P=0·001). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
There was no effect of FVPD ingestion on plasma TAG, HDL-cholesterol, total cholesterol or NEFA compared with control drink consumption (data not shown).
Effect of G298T polymorphism on vasodilation
Table 4 shows the distribution of the different G298T genotypes among participants in the chronic and acute studies. In the chronic study laser Doppler iontophoresis measurements were recorded in a subgroup of nineteen participants (seven GG, eleven GT and one TT). In the acute study the genotype distribution of the twenty-four participants was eleven GG, eleven GT and two TT. There were only two individuals with the TT genotype in the acute study and one in the chronic study; because of lack of power these individuals were excluded from any statistical analysis.
Table 4. Distribution of G298T genotypes among subjects participating in studies of the chronic and acute consumption of fruit- and vegetable-puree-based drinksFootnote *

M, males; F, females.
* For details of studies, see text.
The increase in endothelium-dependent vasodilation induced by acetylcholine after 6 weeks of FVPD consumption compared with the control intervention when all the subjects (seven control and eleven FVPD) were combined did not reach significance (P=0·079), and there was no significant effect of treatment when the genotypes were examined individually (Fig. 5). There was a significant time×treatment effect (P<0·05) of acute FVPD consumption for the GG genotype. After post-hoc tests there was significantly higher endothelium-dependent vasodilation after 180 min (Fig. 6; P=0·028). There was no effect of FVPD on endothelium-dependent vasodilation for individuals with the GT genotype. There was no effect of FVPD on endothelium-independent vasodilation induced by sodium nitroprusside (data not shown).
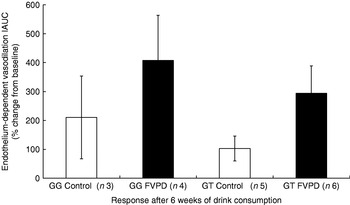
Fig. 5. Endothelium-dependent vasodilation response to acetylcholine of participants with GG (n 7) and GT (n 11) genotype after 6 weeks of intervention with either control (50 ml lemon or orange barley water (Robinsons Ltd, Chelmsford, Essex, UK) diluted to 400 ml with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK) daily; □) or fruit- and vegetable-puree-based drinks (FVPD; one 100 ml bottle of apple, carrot and strawberry and one 100 ml bottle of orange, banana and carrot (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany) daily; ▪).Values are means with their standard errors represented by vertical bars. Data are shown as the percentage from baseline of the incremental area under the curve (IAUC) of the repeated scans by laser Doppler imaging with iontophoresis.

Fig. 6. Endothelium-dependent vasodilation response to acetylcholine of participants (n 22) with GG (□, ![]() ; n 11) and GT (
; n 11) and GT (![]() , ▪; n 11) genotype after acute ingestion of either control (50 ml lemon barley water (Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); □,
, ▪; n 11) genotype after acute ingestion of either control (50 ml lemon barley water (Robinsons Ltd, Chelmsford, Essex, UK), which was matched for sugar composition and diluted with mineral water (The Buxton Mineral Water Co. Ltd, Buxton, Derby., UK); □, ![]() ) or fruit- and vegetable-puree-based drinks (FVPD; 400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany);
) or fruit- and vegetable-puree-based drinks (FVPD; 400 ml apple, carrot and strawberry (Vie shots; Unilever Bestfoods GmbH, Ansbach, Germany); ![]() , ▪). Values are means with their standard errors represented by vertical bars. Data are shown as the percentage from baseline of the incremental area under the curve (IAUC) of the repeated scans by laser Doppler imaging with iontophoresis. The time×treatment effect was significant for GG individuals (P=0·05). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
, ▪). Values are means with their standard errors represented by vertical bars. Data are shown as the percentage from baseline of the incremental area under the curve (IAUC) of the repeated scans by laser Doppler imaging with iontophoresis. The time×treatment effect was significant for GG individuals (P=0·05). The between-treatment effect was significant (after post-hoc tests): *P<0·05.
Discussion
The objective of the present research was to investigate the chronic and acute effects of FVPD consumption on vasodilation, bioavailability of phytochemicals and ascorbic acid and risk factors for CVD. The chronic consumption of FVPD resulted in an increase in dietary carotenoids and ascorbic acid. The significant increase in plasma carotenoids, notably α- and β-carotene, showed that the dietary carotenoids present in the FVPD were absorbed after ingestion. There was no effect of the FVPD on antioxidant status or markers of oxidative stress. It is speculated that this lack of findings could have reflected the preference of the participants to consume the FVPD in the morning, approximately 24 h before they gave a fasted blood sample and morning urine sample. Thus, many of the components of the FVPD would have been metabolised and would no longer be present in the plasma, having limited effect on the antioxidant status or markers of oxidative stress.
Consequently, a second study was performed which involved acute ingestion of FVPD, with blood samples were taken throughout the study day. The participants followed a low-flavonoid diet for the 5 d preceding the study day to ensure there was a low background level of these phytochemicals present in the plasma before FVPD consumption. There was an increase in antioxidant status during the first 4 h after FVPD consumption (data not shown), which was consistent with previous studies(Reference Felgines, Talavera and Gonthier87). The participants displayed a lower glucose and insulin peak concentration after consumption of the FVPD compared with the sugar-matched control. The FVPD was prepared from concentrated juices and purees, so there may have been an effect of the fruit and vegetable matrix on the availability of the sugars present in the FVPD compared with the control drink in which the sugars were in solution in mineral water. There was an increase in plasma ascorbic acid throughout the day following FVPD consumption, which could have contributed to the increase in antioxidant status. Total plasma nitrate and nitrite was also increased following FVPD and remained higher throughout the day compared with the control. The increase in plasma nitrate and nitrite may have been a result of increased NO production or increased NO sparing. The FVPD was a potential source of flavonoid compounds, particularly (–)-epicatechin derived from the 56% (v/v) apple content, which has been shown to increase eNOS activity by scavenging superoxide(Reference Schroeter, Heiss and Balzer76).
After the two studies had been completed the participants were retrospectively genotyped for the G298T polymorphism in the eNOS gene. After chronic consumption of FVPD there was an increase in endothelium-dependent vasodilation induced by acetylcholine, but there was insufficient power to test the effect of genotype. In the acute study there was a significant increase in endothelium-dependent vasodilation in participants with the GG genotype 180 min after FVPD consumption. There was no effect of FVPD consumption on endothelium-dependent vasodilation in participants with the GT genotype or on endothelium-independent vasodilation for either genotype. This result is comparable with findings from venous occlusion plethysmography(Reference Godfrey, Chan and Cassidy88), which have shown that the change in forearm blood flow ratio between the infused and the control arm in response to acetylcholine is decreased in healthy individuals with the GT genotype compared with those with GG variants (3·79 (sd 2·28), P=0·07 v. 3·97 (sd 1·90), P=0·04 respectively) and that there is no effect of genotype on the response to the endothelium-independent vasodilator sodium nitroprusside.
In conclusion, the current studies suggest that consumption of the equivalent of five portions of fruit and vegetables in the form of puree and fruit juice concentrates increases dietary phytochemicals and micronutrients when consumed chronically and increases dietary phytochemicals, micronutrients, plasma antioxidant status and total nitrate and nitrite concentrations when consumed acutely. FVPD consumption has a beneficial effect on endothelium-dependent vasodilation for individuals with the GG variant of the eNOS gene. This research supports previous data suggesting that the G298T polymorphism is associated with reduced endothelium-dependent vasodilation in healthy subjects that is not affected by fruit and vegetable consumption. Further research is required to investigate the effects of genotype in relation to chronic fruit and vegetable consumption and endothelium-dependent vasodilation.
Acknowledgements
The authors acknowledge funding from Unilever Bestfoods (Germany) and the University of Reading Research Endowment Trust Fund. The authors declare no conflict of interest.












