Iodine deficiency during pregnancy is linked to impaired brain development and has been noted as the greatest preventable cause of brain damage(1). These effects are driven through the role of iodine in thyroid hormone production (thyroxine (T4) and tri-iodothyronine), and the subsequent role of thyroid hormone in neurological development. Thyroid hormone production, regulated by thyroid-stimulating hormone (TSH), involves the uptake of circulating iodide into the thyroid, oxidation (by thyroid peroxidase and in the presence of hydrogen peroxide) and attachment to tyrosyl residues on the thyroid-specific protein thyroglobulin. This forms mono-and diiodotyrosine, thyroid peroxidase then couples these to form either tri-iodothyronine or T4, which can be released into the circulation(Reference Zimmermann2).
As the consequences of severe deficiency are considerable, including a negative impact on the economic potential of countries, there have been enormous global efforts to eradicate iodine deficiency(1). While complete eradication of iodine deficiency has not yet been achieved, there has been a marked reduction in the number of countries classified as iodine deficient(3), and a shift towards a classification of mild-to-moderate iodine deficiency rather than severe deficiency(3). It is at this end of the iodine deficiency spectrum that evidence for effects on offspring cognition is limited, but in the past decade a number of studies have added to the evidence base. The picture is now emerging that even mild-to-moderate iodine deficiency during pregnancy may be associated with subtle impairments in cognition and school performance, although there is still a lack of good-quality evidence from randomised controlled trials (RCT) in such regions.
Global picture: what is the iodine status of pregnant women?
The iodine status of pregnant women in the population is based on comparing the median urinary iodine concentration (UIC) measured in spot-urine samples (from a sample of the population) against reference thresholds set by the WHO, UNICEF and the Iodine Global Network(1). This method can classify the group overall but cannot determine the proportion of the population with iodine deficiency. Furthermore, the method is only suitable for populations and large groups, not for assessment of individual pregnant women; in fact there is no suitable biomarker for measuring iodine status in an individual. Iodine deficiency in the pregnant population is indicated by a median UIC <150 µg/l, whereas the threshold is 100 µg/l in school children and adults, reflecting the higher intake recommendations in pregnancy(1). According to WHO, the iodine intake recommendation is for 250 µg/d for pregnant and lactating women, compared with 150 µg/d in the non-pregnant adult(1). The European Food Safety Authority recommendations for pregnancy are slightly lower at 200 µg/d(4).
According to the 2017 Iodine Global Scorecard, insufficient iodine intake is widely present in pregnant women(3). There are seventy-two countries with iodine status data in pregnant women (122 with no data), and thirty-nine (54·2 %) of those are classified as having inadequate iodine status(Reference Gizak, Rogers and Gorstein5); within Europe the percentage of countries with deficiency is higher, 72·4 % (n 21) of the twenty-nine European countries with available data are deficient in pregnancy, including the UK(3). It is often the case that in a population that is classified as iodine-sufficient according to data from school-aged children, pregnant women are classified as iodine-deficient; for example, this is the case in the UK(3, Reference Bath6).
Iodine and brain development
Thyroid hormones are required for brain development and this is particularly relevant in early pregnancy before the onset of fetal thyroid function from mid-gestation(Reference Williams7). In fact it is maternal T4 that is required, as this crosses the placenta and is locally converted to the active form tri-iodothyronine, which then activates thyroid-responsive genes that control brain development; low T4, or hypothyroxinaemia is linked to disordered brain development and functional deficits in the offspring(Reference Velasco, Bath and Rayman8, Reference Moog, Entringer and Heim9). Thyroid hormones are required for neurogenesis, axon and dendrite growth, synapse formation, myelination, and importantly, neuronal migration. Neuronal migration ensures that neurones reach the correct layer of the brain, thus ensuring the correct brain structure; maternal thyroid hormone deficiency has been shown to lead to altered brain structure through incorrect neuronal migration(10). Several specific genes are known to be thyroid-hormone responsive, for example those involved in the expression of reelin (required for neuronal migration) and for myelin basic protein (required for myelination)(10).
Severe iodine deficiency during pregnancy can cause a condition known as cretinism, which is characterised by considerable learning difficulties in the child, uncontrolled movements of arms and legs, and problems with hearing (including deafness) and speech(Reference Chen and Hetzel11). These effects demonstrate the very serious consequences of iodine deficiency on the developing brain. The evidence linking severe iodine deficiency to cretinism comes from trials of iodine supplementation of women in Papua New Guinea where it was shown that iodine supplementation prevented cretinism if iodine was given prior to conception(Reference Pharoah12–Reference Zhou, Anderson and Gibson14). Furthermore, a study of iodised oil v. control injections in pregnant women in severely iodine deficient women in Zaire (at approximately 28 weeks gestation) found higher psychomotor scores in children born to the mother receiving the iodine than in the control group(Reference Zimmermann13). In support of the benefit of early iodine supplementation are results from a trial in Ecuador where IQ scores were higher in children born to mothers who received iodine prior to pregnancy and also from a trial in China that found higher psychomotor scores in children aged 7 years who were born to mothers given iodised oil before the third trimester than from those given iodine later or after birth(Reference Zimmermann13).
Effects of mild-to-moderate iodine deficiency in pregnancy on child development
Although there is a lack of evidence from RCT in regions of mild-to-moderate iodine deficiency, in the past decade there have been a number of observational studies that have evaluated whether this degree of deficiency in pregnancy is associated with child development (Table 1)(Reference Costeira, Oliveira and Santos15–Reference Abel, Brandlistuen and Caspersen27). The results are mixed but mostly point to poorer neurodevelopmental outcomes in children born to women with maternal iodine deficiency. The studies vary considerably in (i) the age of the neurodevelopmental assessment (from 12 months to 15 years); (ii) the neurodevelopmental tests used; (iii) the method to define iodine deficiency (i.e. UIC, urinary iodine:creatinine ratio or estimates of iodine intake); (iv) the timing of iodine exposure (from first trimester to the whole of pregnancy); and (v) sample size. The quality of the studies also varies; for example, not all studies control for potential confounders(Reference Costeira, Oliveira and Santos15).
Table 1. Observational studies of mild-to-moderate deficiency in pregnancy and associations with neurodevelopmental scores in offspring.
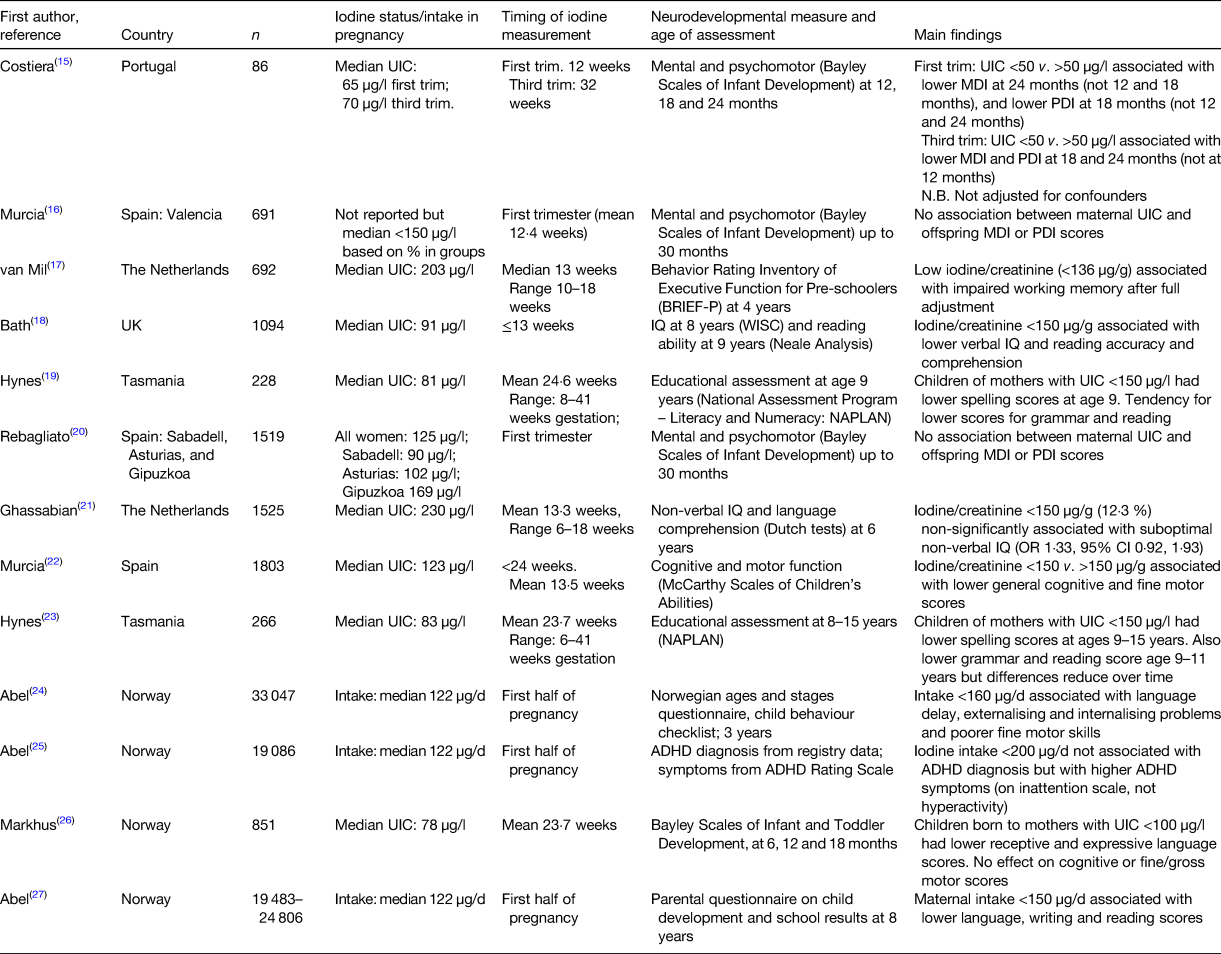
UIC, urinary iodine concentration; MDI, mental development index; PDI, psychomotor development index.
In 2013, two studies were published that evaluated cognitive outcomes in the child up to age 9 years in relation to mild-to-moderate iodine deficiency. Using data and stored first-trimester urine samples from the Avon Longitudinal Study of Parents and Children(Reference Bath, Steer and Golding18) we were the first to examine the relationship between maternal iodine status and child IQ in mild-to-moderate deficiency. We found that children born to mothers with urinary iodine:creatinine ratio <150 µg/g were more likely to have scores in the bottom quartile for verbal IQ, reading accuracy and reading comprehension than those born to women with urinary iodine:creatinine ratio ≥150 µg/g, even after adjustment for twenty-one confounders. Furthermore, we found a dose–response effect when we sub-divided the <150 µg/g group into <50 and 50–150 µg/g, with increasing scores across the three groups of iodine status. The other study that was published in 2013 was from the Gestational Iodine Cohort in Tasmania. That study used educational measures (literacy, numeracy) at age 9 years as the outcome, and found that children born to women with UIC <150 µg/l (i.e. uncorrected for creatinine concentration) had lower spelling scores after full adjustment than those from mothers with UIC ≥150 µg/l. The Gestational Iodine Cohort is unique in that the period of exposure to iodine deficiency was confined to pregnancy because the children grew up in an iodine-replete environment (as a result of iodine fortification of bread in the area)(Reference Hynes, Otahal and Hay19). A follow-up study examined whether the effects seen at age 9 years continued into adolescence and found that the difference in spelling scores between the <150 and ≥150 µg/l group persisted up to age 15 years, even after full adjustment for confounders (including UIC in the child at the age of the test)(Reference Hynes, Otahal and Burgess23). There was also evidence that grammar and reading, but not writing or numeracy, scores were lower in the <150 µg/l group at ages 9 and 11 years, but the effect did not persist up to age 15 years and furthermore the differences in reading scores reduced over time(Reference Hynes, Otahal and Burgess23).
The method of defining iodine deficiency in pregnancy differed across the nine studies and included measures of both iodine status and dietary intake. Iodine status was based on urinary iodine excretion and in some studies, creatinine correction was applied to the UIC(Reference van Mil, Tiemeier and Bongers-Schokking17, Reference Bath, Steer and Golding18, Reference Ghassabian, Steenweg-de Graaff and Peeters21, Reference Murcia, Espada and Julvez22, Reference Markhus, Dahl and Moe26), whereas others only classified mothers on the basis of the UIC(Reference Murcia, Rebagliato and Iniguez16, Reference Hynes, Otahal and Hay19, Reference Rebagliato, Murcia and Alvarez-Pedrerol20, Reference Hynes, Otahal and Burgess23). Creatinine correction is thought to be preferable as the iodine:creatinine ratio is a better proxy for individual iodine status in adults without protein malnutrition and is preferable when used in a cohort of the same sex and limited age range(Reference Knudsen, Christiansen and Brandt-Christensen28), as is the case in studies of pregnant women. Indeed, in the INMA cohort study in Spain, it was only when UIC was corrected for creatinine that associations were shown with cognitive outcomes in the child aged 4–5 years(Reference Murcia, Espada and Julvez22) and the earlier INMA studies did not find associations with maternal UIC and younger child outcomes(Reference Murcia, Rebagliato and Iniguez16, Reference Rebagliato, Murcia and Alvarez-Pedrerol20). Conversely, the Little In Norway study found significant associations with language scores when using UIC but not with the iodine:creatinine ratio(Reference Markhus, Dahl and Moe26). We have previously shown that UIC in a study of UK pregnant women was very low, and this was related to the fact that we recruited women at their ultrasound appointment where they had been instructed to attend with a full bladder(Reference Bath, Furmidge-Owen and Redman29). This methodological bias may influence classification of individuals if the UIC is used, rather than the iodine:creatinine ratio, although further research in this area is required. Dietary iodine intake was estimated in the Norwegian birth cohort, MoBA, and while there are limitations to the assessment of iodine intake, the FFQ was comprehensive (255 items) and was validated against biomarkers and 4 d food diaries(Reference Abel, Caspersen and Meltzer24, Reference Abel, Ystrom and Caspersen25, Reference Abel, Brandlistuen and Caspersen27). One of the problems with urinary iodine excretion is that it may not reflect habitual iodine intake as it reflects only the short term, while intake from a FFQ may give a better long-term measure of iodine exposure in pregnancy and therefore the results from the MoBa studies(Reference Abel, Caspersen and Meltzer24, Reference Abel, Ystrom and Caspersen25, Reference Abel, Brandlistuen and Caspersen27) give useful additional information about the effect of iodine on child development.
The underlying iodine status of the population may be important when interpreting the results of the observational studies. Two of the studies outlined in Table 1 were conducted using data from Generation R in the Netherlands and although they examined the effect of low iodine status, overall the women were classified as iodine-sufficient(Reference van Mil, Tiemeier and Bongers-Schokking17, Reference Ghassabian, Steenweg-de Graaff and Peeters21). Furthermore, living in an iodine-sufficient country, it is likely that women had thyroidal stores of iodine to draw upon and maintain thyroid hormone production.
Many of the studies in regions of mild-to-moderate deficiency seem to show that iodine deficiency in pregnancy affects verbal IQ rather than performance IQ(Reference Bath, Steer and Golding18, Reference Moleti, Trimarchi and Tortorella30), although one study did find an association with non-verbal IQ(Reference Murcia, Espada and Julvez22). It is suggested that lower verbal IQ may be caused by difficulties in processing auditory information in the central nervous system(Reference Hynes, Otahal and Burgess23), and in the study by Hynes et al., the results suggested that working memory and auditory processing speed is affected by iodine deficiency in pregnancy(Reference Hynes, Otahal and Burgess23). This fits with results from iodine supplementation studies in school-aged children(Reference Gordon, Rose and Skeaff31, Reference Zimmermann, Connolly and Bozo32) where the results showed no improvement on tests that rely on working memory, suggesting that the effects of iodine deficiency in pregnancy cannot be overcome by adequacy in childhood, and therefore (the Wechsler Intelligence Scale for Children) represents permanent damage to the brain(Reference Hynes, Otahal and Burgess23).
Consideration of the neurological tests used to examine the associations with iodine deficiency is important as it may be that global assessment tests commonly used, such as the Bayley Scales of Infant Development or WISC, are not sensitive to the effects of iodine or thyroid deficiency(Reference Bell, Ross and Goodman33). Reviews in this area point to the fact that prenatal exposure to thyroid hormone is linked to visuospatial, motor, visuomotor(Reference Zoeller and Rovet34) and that applying tests of the visual pathway in children would be important in future studies of iodine in pregnancy(Reference Bell, Ross and Goodman33).
The effect of iodine supplementation during pregnancy on child development
In 2015, a systematic review of eight studies concluded that RCT in regions of severe deficiency have shown reduced incidence of cretinism and improvements in child motor function with iodine supplements commenced prior to, or in early pregnancy(Reference Zhou, Anderson and Gibson14). However, there was no effect on general cognition, growth or pregnancy outcomes, although the quality of evidence was not high. Furthermore, they found that evidence for effects on child outcomes in mild-to-moderate deficiency was lacking as the six RCT(Reference Romano, Jannini and Pepe35–Reference Antonangeli, Maccherini and Cavaliere40) in such regions only evaluated the effect of iodine supplementation during pregnancy on maternal and infant thyroid function(Reference Zimmermann41).
A later Cochrane review included eleven trials and found no clear evidence for either benefit or harm of iodine supplementation in pregnancy when considering maternal and infant thyroid function, as well as child neurodevelopment(Reference Harding, Pena-Rosas and Webster42). The review included two RCT with child outcomes; both found no difference in cognitive scores between the iodine and control group, but both were also underpowered(Reference Brucker-Davis, Ganier-Chauliac and Gal43, Reference Zhou, Skeaff and Ryan44). The first trial, in France (median UIC 103–111 µg/l), randomised 111 women from the first trimester (median 10 weeks) to receive prenatal supplements with/without 150 µg iodine daily, and all women received dietetic advice on optimising iodine intake(Reference Brucker-Davis, Panaia-Ferrari and Gal45). Only forty-four children had neurocognitive assessments at age 2 years and there was no difference in scores between the iodine (n 19) and control group (n 22)(Reference Brucker-Davis, Ganier-Chauliac and Gal43). The second trial was the aborted trial in Australia and New Zealand, the Pregnancy Iodine and Neurodevelopment in Kids trial (PINK), which was stopped after fifty-nine women were recruited in Australia (planned sample size of 1098) at a mean gestational age of 33 weeks (mean 16 weeks of treatment). The trial was stopped because funding was withdrawn(Reference Zhou, Skeaff and Ryan44) as the National Health Medical Research Council, introduced a recommendation for pregnant and lactating women to take a supplement with 150 µg iodine(46), and therefore it was felt that the trial was in conflict with the recommendation. The fact that the recommendation to take iodine was introduced prior to the evidence from the trial is unfortunate, and underpins the difficulty that may be faced when trying to conduct similar trials in other countries that have already introduced recommendations, such as in the USA and Canada(Reference Stagnaro-Green, Abalovich and Alexander47).
Both the 2015 systematic review and the Cochrane review were conducted prior to the publication of the first randomised, placebo-controlled trial in mildly deficient pregnant women. The long-awaited MITCH (Maternal Iodine Supplementation and Effects on Thyroid Function and Child Development) trial randomised pregnant women in India and Thailand (median UIC 131 µg/l) to 200 µg iodine/d or placebo from ≤14 weeks gestation to the end of pregnancy and assessed cognition in the offspring aged 5–6 years. There was no difference in any measure of child cognition or behaviour scores between the groups(Reference Gowachirapant, Jaiswal and Melse-Boonstra48). However, there are several key points and limitations that need to be taken into consideration before this trial is considered to be the definitive answer to whether mild-to-moderate iodine deficiency affects cognition, or indeed whether pregnant women in such areas should be advised to take an iodine supplement during pregnancy(Reference Bath49). First, the group of women in India were not classified as iodine deficient at baseline (median 188 µg/l) and therefore would not have been expected to benefit from the iodine supplement; the authors did perform stratified analyses and there was no effect of iodine on child cognition group from Thailand who were iodine deficient (median UIC 112 µg/l), although the statistical power would have been lower in sub-group analyses. Secondly, the trial was conducted in regions with established iodised salt programmes, and the WHO recommendation is that supplements are not required in such regions as women should enter pregnancy with adequate iodine stores(Reference Andersson and de Benoist50). Indeed this is suggested by the relatively low (<10 µg/l) thyroglobulin concentration in the women at baseline which does not indicate long-term deficiency. Thirdly, iodine status increased in the placebo group to the lower end of the adequate range, which may have reduced any effect of iodine. Also of note is the fact that the dose of iodine was relatively high at 200 µg daily; other research, although observational, has suggested a negative effect on maternal thyroid function(Reference Rebagliato, Murcia and Espada51) and child cognitive scores(Reference Murcia, Rebagliato and Iniguez16, Reference Rebagliato, Murcia and Alvarez-Pedrerol20) with daily doses of ≥150 µg iodine, although this evidence was not available when the trial was planned (prior to 2008) and there was no suggestion of any harm of the supplements in the MITCH trial. Given these limitations, further RCT evidence is required to know whether mild-to-moderate iodine deficiency affects brain development and child cognition.
To that end, a trial is underway in Sweden that will randomise a total of 1275 women (median 110–111 µg/l)(Reference Manousou, Eggertsen and Hulthen52) ≤12 weeks gestation to a supplement with/without 150 µg iodine daily and follow the children to age 7 years(Reference Manousou, Johansson and Chmielewska53). However, the trial is limited by the fact the study is using commercially available supplements and therefore each arm of the trial differs by more than just the iodine content; the placebo group is a multivitamin supplement, while the intervention arm contains iron (12 mg), selenium (50 µg) and a higher dose of folic acid than the placebo group (400 v. 200 µg). Iron and selenium are also required for thyroid hormone production and function, which may therefore affect maternal and infant thyroid function and confound the effects of iodine on child development. Furthermore folic acid has recently been shown to have effects on child neurodevelopment when supplementation continued throughout pregnancy and this may further complicate interpretation of the trial(Reference Henry, Cassidy and McLaughlin54).
There is a pressing need for an RCT of iodine supplementation in early pregnancy in areas of moderate iodine deficiency (i.e. median UIC 50–100 µg/l) that includes cognitive outcomes in the child. Time may be limited for this trial as more countries introduce recommendations for pregnant women to take iodine supplements. For example, in recent years recommendations have been introduced for pregnant women to take iodine supplements in Portugal(55), Norway (for those with low intake of milk and dairy products)(56, Reference Henjum, Lilleengen and Aakre57) and Israel(58) and therefore it may become politically and ethically more difficult to run trials in such countries. Such a trial may still be possible in the UK where there are no such recommendations, no advice for pregnant women to increase iodine intake, and a lack of an iodised salt policy(Reference Bath, Jolly and Rayman59). However, as time passes, more women may become iodine-aware or take a prenatal supplement that contains iodine (even if not selected for this reason); in the aborted PINK trial, 51 % of women who did not meet the inclusion criteria were excluded because they were already taking a supplement containing iodine(Reference Zhou, Skeaff and Ryan44).
Effects of iodine supplementation: evidence from other intervention studies
There are three intervention studies in mildly-to-moderately deficient pregnant women with neurological outcomes in the offspring(Reference Berbel, Mestre and Santamaria60–Reference Santiago, Velasco and Muela62), all were conducted in Spain but none were randomised placebo-controlled trials (Table 2). In fact in one of the studies, it was considered unethical to have a placebo group and therefore comparisons were made with women who reported that they had not taken iodine during the pregnancy(Reference Velasco, Carreira and Santiago61). In the study by Berbel and colleagues it is important to note that the groups differed not only by the length of iodine supplementation in pregnancy, but also by maternal thyroid function as women were only included in group 1 if their free T4 (fT4) value was >20th percentile at enrolment, and were included in groups 2 and 3 if they were hypothyroxinaemic (fT4 <10th percentile and normal TSH) at enrolment(Reference Berbel, Mestre and Santamaria60). Therefore, the results may reflect differences in fT4 in early pregnancy rather than the effect of delayed iodine supplementation. Although this study is often cited as evidence for a benefit of iodine supplementation, particularly if started in the first trimester, it has considerable limitations and therefore does not provide strong evidence. In the 2009 study by Velasco et al., children born to mothers who received iodine supplements have significantly higher psychomotor scores than the controls(Reference Velasco, Carreira and Santiago61). In addition, several items on the Behaviour Rating Scale showed that behaviour of the children in the intervention group was more in line with their age than the control group for: reaction to the mother/other persons, producing sounds by banging, cooperation, activity and arousal(Reference Velasco, Carreira and Santiago61). However, it is important to point out that there were significant differences between the iodine and control groups, including the age of neurological testing in the child (5·47 and 12·44 months, respectively), although this should be accounted for by the fact that they used the development index, rather than raw scores. The third intervention study in Spain did not have a placebo group but randomised women to three treatments with differing doses of iodine; either advice to use iodised salt, or supplements of 150 or 230 µg iodine (as 200 and 300 µg potassium iodide)(Reference Santiago, Velasco and Muela62). There was no difference between the three groups in terms of maternal thyroid function on neurodevelopmental outcomes; however, the sample size was relatively small and the study did not include a placebo group.
Table 2. Intervention studies in Spain that gave iodine during pregnancy and measured neurodevelopmental outcomes in the child.
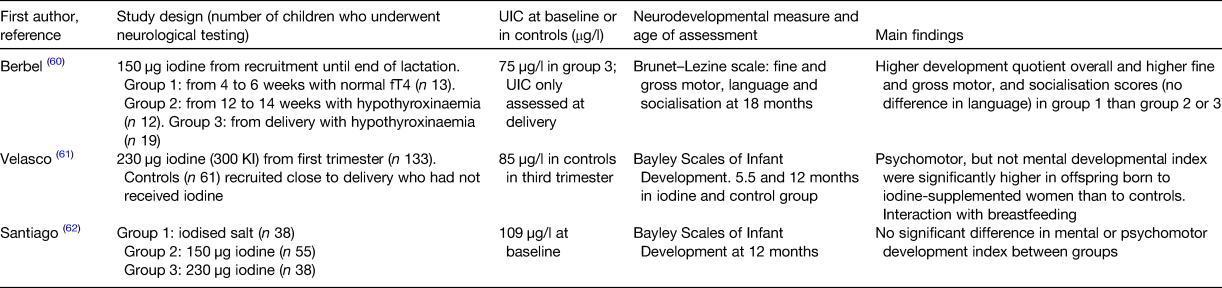
UIC, urinary iodine concentration; fT4, free thyroxine.
Are there any risks associated with use of iodine supplements in pregnancy?
There is some weak evidence that commencing a supplement during pregnancy is associated with adverse effects on maternal thyroid, or infant neurodevelopment. In a cross-sectional study in three areas of Spain (Valencia, Gipuzkoa (Basque Country) and Sabadell (Catalonia)) that are part of the INMA cohort study (overall median UIC 137 µg/l), intake of iodine supplements containing ≥200 µg was associated with greater odds of TSH >3 µU/ml than use of supplements with <100 µg iodine (OR 2·51, 95% CI 1·16, 5·43)(Reference Rebagliato, Murcia and Espada51). It is important to note that in Sabadell and particularly in Valencia, most women took iodine as part of a multivitamin-mineral preparation, while in Gipuzkoa the majority of women were taking potassium iodide. A follow-up study of mother–child pairs from the Valencia cohort (n 691) evaluated the association between supplement taking and neurodevelopment at age 12 months(Reference Murcia, Rebagliato and Iniguez16). That study found a negative association between iodine supplements of ≥150 µg/d and psychomotor development scores (OR for psychomotor development scores <85: 1·8, 95% CI 1·0, 3·3 compared with iodine supplement <100 µg/d), but no association with mental development scores(Reference Murcia, Rebagliato and Iniguez16). However, as noted above, the women in Valencia mostly took iodine via multivitamin and mineral supplements and therefore the evidence for potentially negative effects of iodine on psychomotor development must be taken with caution. Indeed, when the results from three other centres of the INMA cohort study (n 1519) were examined, the picture becomes less clear(Reference Rebagliato, Murcia and Alvarez-Pedrerol20); overall in the additional centres (Gipuzkoa, Sabadell and Asturias), there was no significant effect of an iodine supplement on neurodevelopmental scores (either psychomotor or mental), but there was a negative association in the cohort from Asturias. When results from all four INMA centres were pooled, there was an increased odds of low psychomotor scores (<85) with maternal intake of supplements containing ≥150 µg iodine/d than <100 µg iodine d (OR 1·7, 95% CI 1·1, 2·6). However, this negative effect was driven by the results in Valencia and Asturias and in both of these regions iodine was mostly taken as part of a multivitamin and mineral preparation.
The INMA cohort (all four centres) has recently published results of longer term follow-up of the child and found no relationship between dose of iodine supplement during pregnancy and cognitive and motor development at age 4–6 years(Reference Murcia, Espada and Julvez22). This may suggest that any possible negative effect of iodine-containing supplements on motor development up to age 18 months was transient and not seen at the later assessment, or may also reflect insensitivities in the Bayley Scales of Infant Development compared with the later measure by the McCarthy Scales of Children's Abilities in older children.
A cohort study that measured iodine intake (from FFQ) found that use of iodine supplements was associated with increased risk of attention-deficit/hypersensitivity disorder symptoms (though not attention-deficit/hypersensitivity disorder diagnosis)(Reference Abel, Ystrom and Caspersen25). However, it is important to highlight that this effect was no longer significant when adjusting for use of other nutritional supplements (e.g. folic acid) supplement use in the mother.
The potential negative effects of iodine supplements may be explained by ‘thyroid stunning’ as a result of an abrupt increase in iodine supply from iodine supplements, an idea proposed by Moleti et al. in Italy(Reference Moleti, Di Bella and Giorgianni63). They found that women who reported using iodised salt for 2 years prior to pregnancy (n 105) had higher fT4 and lower TSH throughout pregnancy than women who started using iodine supplements when pregnant (n 168)(Reference Moleti, Di Bella and Giorgianni63). This idea is supported by a recent cross-sectional study in Hungary where women were grouped according to timing of iodine supplementation: those who used iodine supplements for at least 4 weeks prior to pregnancy (n 27) had a lower TSH (1·97 v. 1·72 mU/l) and thyroglobulin (14·5 v. 9·1 µg/l) concentration at 16 weeks gestation than those who started supplements during pregnancy (n 51), although there was no effect on fT4(Reference Katko, Gazso and Hircsu64). This study is limited by the single cross-sectional measure at 16 weeks, and the small sample size in the groups. Thyroid stunning via iodine-induced hypothyroidism is thought to be more likely at high doses of iodine (such to induce the Wolff–Chaikoff effect), rather than the nutritional doses reported in these studies. The exact mechanism and effect of a sudden supply of iodine is therefore unclear and further research in this area is required.
Other evidence suggests that it is not just the iodine supplement and when this commences that matters, but the habitual iodine intake in the individual. In the MoBa cohort study in Norway, there was some suggestion of a negative effect on child neurodevelopment at age 3 years with use of iodine-containing supplements in pregnant women with a low intake of iodine (i.e. <160 µg/d)(Reference Abel, Caspersen and Meltzer24). Children born to women with low iodine intake who took supplements were more likely to have internalising behaviour problems at age 3 years(Reference Abel, Caspersen and Meltzer24). Interestingly there was no evidence of a negative effect in the children born to women who had an intake above 160 µg/d and took iodine-containing supplements, or indeed in those with a low intake but who started the supplement prior to pregnancy(Reference Abel, Caspersen and Meltzer24), which suggests that an abrupt increase in the supply of iodine during pregnancy may be problematic. However, given the observational nature of this study, and the fact that iodine was part of a multivitamin and mineral preparation, the exact mechanism remains unknown. In a later study by the same group, there was no association between supplement use and neurodevelopmental outcomes at age 8 years, or an interaction between supplement use, timing and habitual iodine intake(Reference Abel, Brandlistuen and Caspersen27). Therefore overall, the MoBa data suggest no benefit of iodine supplementation but mixed results with respect to potential negative effects of iodine supplements.
The importance of iodine prior to pregnancy
The thyroid can store iodine, which is important as these stores can then be used during pregnancy to maintain thyroid hormone production. For this reason, adequate iodine intake in women of childbearing age is vital so that thyroidal stores can be maximised. Evidence that this is important comes mainly from observational data but a picture is emerging that iodine prior to pregnancy is as important, or possibly more important, than iodine during pregnancy.
The evidence from the observational studies suggests iodine supply (either use of iodised salt or supplements) prior to pregnancy is associated with lower TSH(Reference Moleti, Di Bella and Giorgianni63–Reference Moleti, Lo Presti and Campolo65), higher fT4(Reference Moleti, Di Bella and Giorgianni63), lower thyroglobulin(Reference Katko, Gazso and Hircsu64) (suggesting a lower thyroid volume) and lower thyroid volume(Reference Santiago, Velasco and Muela62) than either supplementation or iodised salt use, which commenced in pregnancy. More recently, the idea that pre-pregnancy iodine supply is important has been extended to study the relationship between pre-pregnancy iodine status and child cognition in a UK-based cohort study(Reference Robinson, Crozier and Miles66). Using samples and data from the UK-based Southampton Women's Survey (n 651), the relationship between pre-pregnancy urinary iodine excretion (iodine:creatinine ratio) and offspring cognition at the age of 6 years has been explored(Reference Robinson, Crozier and Miles66). The women in the study were classified as iodine-sufficient overall based on the UIC in relation to WHO cut-off for adults (median UIC 108 µg/l v. threshold of 100 µg/l(1)). The study found that pre-conception urinary iodine: creatinine ratio was positively associated with child IQ at age 6–7 years, even after adjustment for potential confounders and maternal IQ; a urinary iodine:creatinine ratio ≤50 µg/g was associated with a 7·5 lower IQ compared with those with values >150 µg/g(Reference Robinson, Crozier and Miles66). It is perhaps important to note that the urine samples were collected a considerable time before conception (median 3·3 years) and therefore assumptions were made that this reflected long-term status in the preconception period. Nevertheless, this is an important study and further work in this area is warranted.
Conclusions
From the evidence reviewed here, it appears that women of reproductive age should ensure adequate iodine intake during pregnancy but preferably in the months prior to pregnancy. Ideally this should be achieved through dietary intake where possible, good sources are seafood (particularly white fish), eggs, and milk and dairy products. In many countries, but not the UK(Reference Bath, Button and Rayman67), iodised salt is also a source of iodine but salt intake should be within salt-reduction recommendations. Those who avoid iodine-rich foods (e.g. vegetarians and vegans), or consume unfortified milk-alternative drinks in place of cows milk(Reference Bath, Hill and Infante68), may be at risk of iodine deficiency and could consider an iodine-containing supplement in order to meet requirements. However, the current evidence suggests that where possible the supplement should be started prior to pregnancy. Furthermore, high-dose iodine-containing supplements (>150 µg/d) should be avoided, and kelp or seaweed supplements should not be used as an iodine source as they can lead to excess intake(Reference Zimmermann and Delange69).
There is a general lack of knowledge of the important of iodine in pregnancy and therefore there is work to be done from a public-health perspective in terms of awareness-raising, both in women of reproductive age, and clinicians such as midwives and general practitioners. For example, in the UK, research in Scotland and Northern Ireland has shown that pregnant women are not given advice about iodine, are not aware of its importance, and also do not know the main dietary sources (<10 % knew that milk and dairy products are sources of iodine)(Reference O'Kane, Pourshahidi and Farren70, Reference Combet, Bouga and Pan71). Even in countries with established recommendations for iodine supplements in pregnancy (such as the USA and Australia and New Zealand), the evidence shows that knowledge is poor, and that many health professionals do not recommend the supplements, or recommend an inappropriate dose(Reference De Leo, Pearce and Braverman72–Reference Charlton, Yeatman and Lucas74). In seems therefore that public-health campaigns are required to raise the profile of iodine in pre-pregnancy and pregnancy, which may help to reduce the negative consequences of iodine deficiency.
Acknowledgements
With thanks to Professor Margaret Rayman and Dr Inés Velasco for helpful discussions.
Financial Support
None.
Conflict of Interest
None.
Authorship
The author had sole responsibility for all aspects of this paper.




