Obesity is associated with hypothalamic(Reference Arruda, Milanski and Coope1, Reference Kreutzer, Peters and Schulte2) and peripheral(Reference McGuire, Brusnahan and Bilek3) inflammation. The hypothalamus is key in the regulation of appetite and energy homeostasis with dysfunction resulting in obesity. Dietary long chain saturated fatty acids (LCSFA) are causative in hypothalamic inflammation(Reference Thaler, Yi and Schur4, Reference De Souza, Araujo and Bordin5) but LCSFAs only appear effective in the presence of sugar via the formation of advanced glycation end products (AGEs)(Reference Gao, Bielohuby and Fleming6). This raises a paradox as n-3 PUFAs, which are anti-inflammatory, are highly susceptible to AGE formation via lipid peroxidation. Thus, we investigated whether AGEs are pro inflammatory in the hypothalamic neuronal cell line, mHypoE-N42 (N42).
N42 cells were treated with 200 μM palmitic (PA), oleic (OA), or eicosapentaenoic (EPA) for 6 hours(Reference Sergi, Morris and Kahn7). N42 cells were also challenged with 100 μM AGEs prepared as described by Castilho et al.(Reference Castilho, Okuda and Pinto8) for up to 24 hours. Gene expression of pro-inflammatory cytokines (IL6 and TNFα) was measured by real-time quantitative RT-PCR using B2m as a reference gene. Statistical analysis was performed by ANOVA followed by Students t -test.
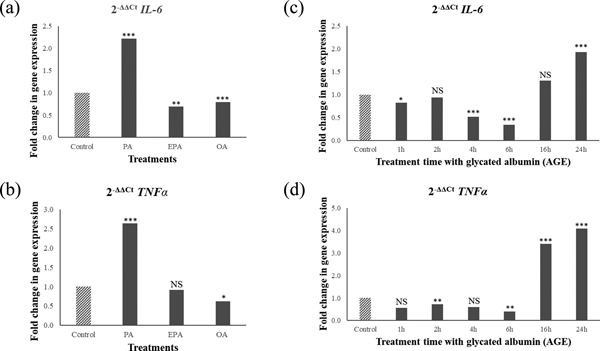
PA upregulated IL6 and TNFα, whereas OA and EPA downregulated these genes (Fig. 1a and 1b). In contrast, AGE challenge downregulated IL6 and TNFα expression up to 6 hours followed by upregulation after 16 hours (Fig.1c and 1d). Gene expression and Western blotting showed the presence of receptor for AGE (RAGE) in cells and media confirming that cells are able to respond to AGEs.
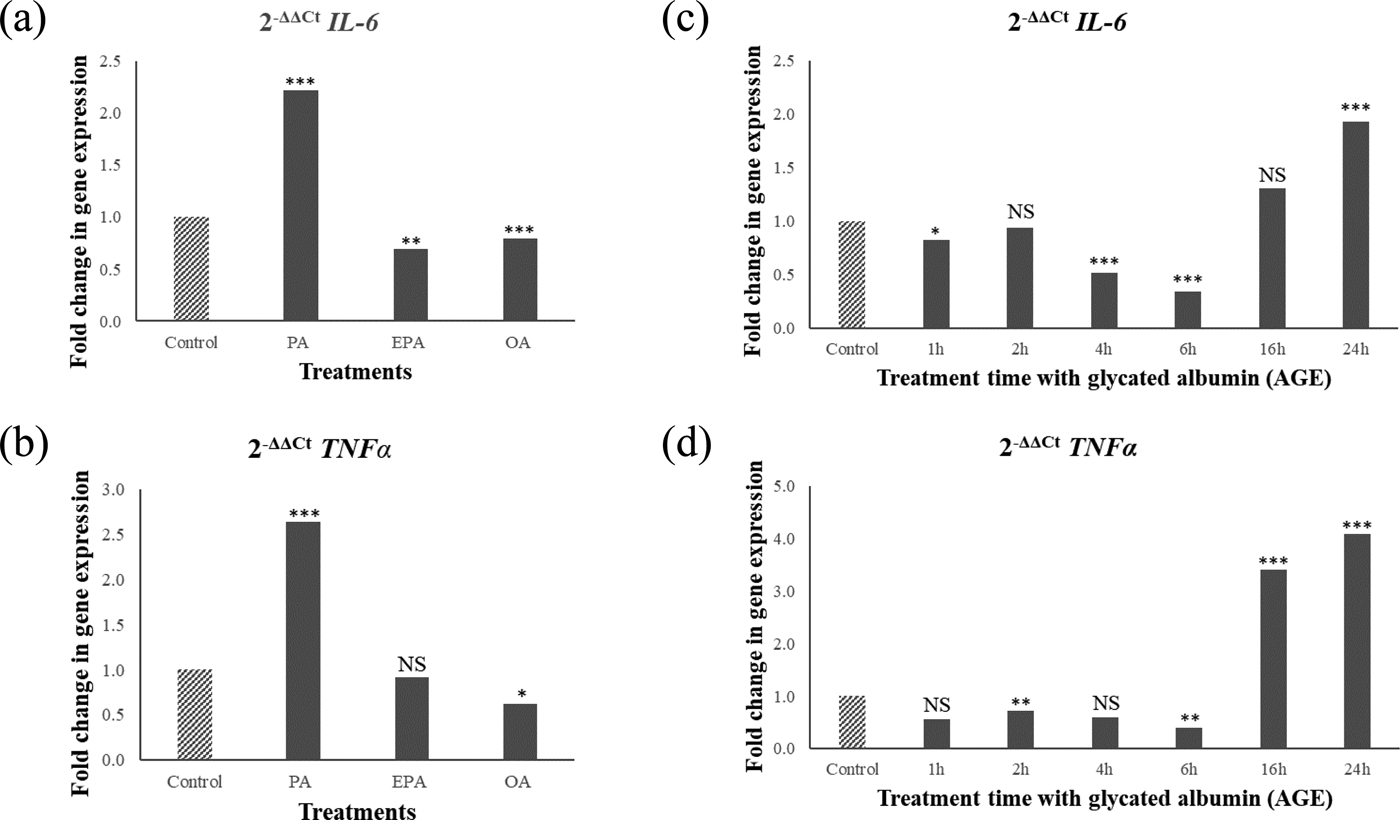
Fig. 1. Relative pro-inflammatory gene expression (IL6 and TNFα) in the hypothalamic neurons after 6 hour fatty acid challenge (1a and 1b) or 1-24 hours AGE challenge (1c and 1d). Values are ratios; stars indicate statistical significance of the differences between normalised threshold cycle number (ΔC t) of control and treatments (n = 4). *p < 0.05; **p < 0.01; ***p < 0.001; NS non-significant.
Taken together, these results suggest that AGEs trigger pro-inflammatory responses in the hypothalamus on a different timescale compared to PA; and the pro-inflammatory effect of PA is not mediated via the formation of AGEs.