Irritable bowel syndrome and the low FODMAP diet
Irritable bowel syndrome (IBS) is a chronic and debilitating functional gastrointestinal (GI) disorder characterised by abdominal pain and altered bowel habit. Global prevalence is reported to be in the region of 5⋅7–11⋅2 % of the population, depending upon definition and region(Reference Lovell and Ford1,Reference Palsson, Whitehead and Van Tilburg2) . The Rome criteria used to define functional bowel disorders recently updated the characteristics used to define IBS. Specifically, IBS is diagnosed when abdominal pain that is related to defaecation (i.e. related to increased or decreased stool frequency or consistency) is experienced at least one day per week. This update in definition removes ambiguity in the former classification that included both abdominal pain ‘or discomfort’ and at a lower frequency of symptoms(Reference Palsson, Whitehead and Van Tilburg2).
Within the Rome IV IBS classification, patients can be subtyped as: diarrhoea predominant (IBS-D, >25 % loose stool and <25 % hard stool); constipation predominant (IBS-C, >25 % hard stool and <25 % loose stool); mixed type (IBS-M, >25 % loose stool and >25 % hard stool); or un-subtyped (IBS-U, <25 % loose stool and <25 % hard stool)(Reference Lacy, Mearin and Chang3). In addition to classifying IBS by predominant stool form, patients can be classified as post-infectious IBS (PI-IBS) where the symptoms began with or shortly after a GI infection and have not resolved within 6 months, inferring chronicity(Reference Lacy, Mearin and Chang3).
National Institute for Health and Care Excellence (NICE) guidelines for managing IBS recommend first-line dietary advice (healthy eating advice focussing on meal frequency, size, composition and intake of fat, spicy foods, caffeine, fluids and fibre), and if this fails to resolve symptoms, second-line dietary advice should be followed (the low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet (LFD))(4). The LFD involves the restriction of fermentable oligosaccharides, disaccharides, monosaccharides and polyols, including fructans (e.g. wheat, onion, garlic), galacto-oligosaccharides (GOS, e.g. lentils, beans, peas, cashews), lactose (e.g. milk), fructose in excess of glucose (e.g. mango, honey), sorbitol (e.g. avocado, apple, broccoli) and mannitol (e.g. celery)(Reference Whelan, Martin and Staudacher5).
The LFD consists of three stages. Stage 1 (restriction) involves the restriction of all FODMAP sources and is recommended for a minimum of 4 weeks followed by the evaluation of symptom response. Stage 2 (reintroduction) involves challenge with increasing doses of individual FODMAP while continuing restriction of all other FODMAP. Specific challenges include individual foods containing fructans, GOS, lactose, fructose, sorbitol and mannitol, to identify the classes and amount of FODMAP that trigger symptoms in that patient. Stage 3 (personalisation) involves patients consuming liberalised types and amounts of FODMAP-containing foods that were well tolerated during stage 2, while those inducing significant GI symptoms continue to be excluded, with the aim of managing GI symptoms in the long term.
One of the first reports of the role of some FODMAP in IBS symptom induction was a double-blind, quadruple cross-over, placebo-controlled, re-challenge trial in twenty-six patients with IBS who had previously achieved adequate relief of their symptoms by following an LFD(Reference Shepherd, Parker and Muir6). Fewer patients reported adequate relief of abdominal symptoms when challenged with fructans, fructose or a mixture of both, compared to glucose (control). Both fructans and the mixture of fructans and fructose led to significantly greater severity of abdominal pain, bloating and flatulence than placebo and fructose led to significantly worse pain and bloating severity than placebo(Reference Shepherd, Parker and Muir6).
Mechanistically, ingestion of fructose leads to an increase in small bowel water peaking at 75 min after ingestion and fructans lead to increased colonic gas peaking at 3–5 h after ingestion(Reference Murray, Wilkinson-Smith and Hoad7). The effect of fructose on small bowel water can be ameliorated by glucose co-administration that leads to increased fructose absorption in the small bowel via a glucose–fructose transporter complex (GLUT2). However, when trialled in IBS, the addition of glucose to fructose solutions did not prevent symptom induction despite reducing breath hydrogen production compared with fructose alone(Reference Tuck, Ross and Gibson8). Further, fructan-induced colonic gas production leads to similar gas production in IBS and healthy controls, despite a peak in symptom intensity in IBS(Reference Major, Pritchard and Murray9) suggesting hypersensitivity to gas production is responsible for IBS symptoms, rather than greater gas production per se. The altered hypersensitivity may be due to varied microbial signalling, disordered tight junction proteins or heightened GI immune signalling and subsequent inflammatory response or a combination of all these components(Reference Deiteren, de Wit and van der Linden10).
The first randomised controlled trial (RCT; n 41) of LFD advice found that it resulted in significantly higher adequate relief of IBS symptoms compared with habitual diet(Reference Staudacher, Lomer and Anderson11). Subsequently, numerous studies have confirmed that the LFD alleviates IBS symptoms compared to habitual diet(Reference Harvie, Chisholm and Bisanz12), typical diet(Reference Halmos, Power and Shepherd13,Reference Chumpitazi, Cope and Hollister14) , a high FODMAP diet(Reference McIntosh, Reed and Schneider15) or a sham diet(Reference Staudacher, Lomer and Farquharson16) (Table 1).
Table 1. Randomised controlled trials and randomised comparative trials of the low FODMAP diet in irritable bowel syndrome (IBS) and the effect on global and individual symptoms
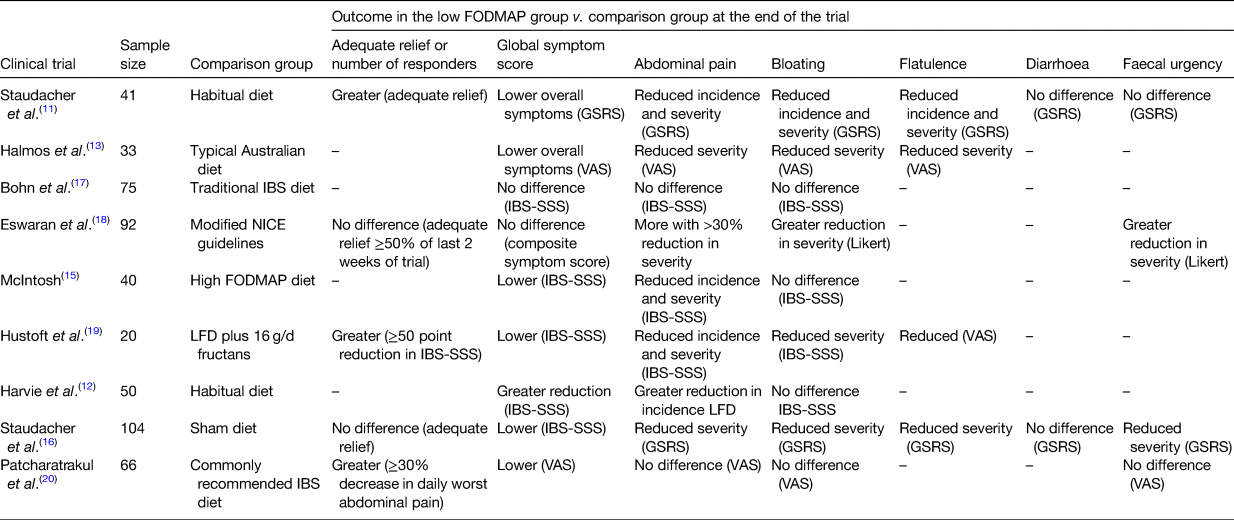
LFD, low FODMAP diet; GSRS, gastrointestinal symptom rating scale; VAS, visual analogue scale; IBS, irritable bowel system; IBS-SSS, IBS severity scoring system.
There are now numerous systematic reviews and meta-analyses of clinical trials of the LFD showing improvements in global symptoms (relative risk = 0⋅69; 95 % CI 0⋅54, 0⋅88; P = 0⋅003)(Reference Dionne, Ford and Yuan21), overall GI symptoms (standardised mean difference = −0⋅62; 95 % CI −0⋅93, −0⋅31; P = 0⋅0001), abdominal pain (standardised mean difference = −0⋅50; 95 % CI −0⋅77, −0⋅22; P = 0⋅008)(Reference Schumann, Klose and Lauche22) and bloating (OR 1⋅75, 95 % CI 1⋅07, 2⋅87; P = 0⋅03)(Reference Marsh, Eslick and Eslick23).
Therefore, the LFD is an evidence-based dietary therapy that can be used when first-line healthy eating approaches have failed to reduce symptoms. However, there are several challenges associated with the use of this diet. The aim of this review is to critically discuss the challenges of the LFD faced by patients, health professionals and the wider healthcare service and to discuss research findings and practical guidance for how these challenges can be minimised and mitigated, so that the clinical effectiveness of the LFD can be realised in practice.
Challenge 1: the low FODMAP diet affects the luminal microbiota
Despite the beneficial effects of the LFD on global and specific symptoms, modifications in the GI microbiota have been identified. The first RCT of the LFD demonstrated it lowered bifidobacteria compared with habitual diet(Reference Staudacher, Lomer and Anderson11), whilst two further RCT have demonstrated a lower absolute abundance of bifidobacteria when comparing the LFD with placebo diets(Reference Staudacher, Lomer and Farquharson16,Reference Halmos, Christophersen and Bird24) and a fourth RCT did not report the LFD lowered bifidobacteria but rather that a high FODMAP diet increased both Bifidobacteriaceae and some genera within the Lachnospiraceae family(Reference McIntosh, Reed and Schneider15).
In a cross-over feeding study comparing an LFD to typical Australian diet, a number of microbial effects between the two diets were observed including greater Clostridium cluster XIVa following a typical Australian diet, although given that diet was higher in FODMAP than participants' habitual diet, at least some of the differences may be attributed to a prebiotic effect of increasing FODMAP in the typical Australian diet control group(Reference Halmos, Christophersen and Bird24). In comparison to the habitual diet, the same study reported the LFD reduced absolute abundance of total bacteria, Clostridium cluster IV, Faecalibacterium prausnitzii and Bifidobacterium. However, there was no difference between habitual diet and LFD for the abundance of total bacteria(Reference Halmos, Christophersen and Bird24). In a comparison of the LFD with a traditional IBS diet (first-line advice from NICE(4)), the LFD but not traditional IBS diet was shown to specifically reduce Bifidobacterium, Mycoplasma hominis and the phylum Actinobacteria from baseline, suggesting that it is FODMAP restriction that impacts the gut microbiota(Reference Bennet, Bohn and Storsrud25). The consistently reported effect of the LFD in reducing the abundance of bifidobacteria could be considered a potentially negative consequence, although no data are available on the health consequences of the lower bifidobacteria resulting from the LFD nor on the impact on bifidobacteria in the long term following FODMAP reintroduction.
Restricting fermentable substrate for saccharolytic bacteria, and therefore reducing saccharolytic fermentation should logically reduce SCFA production. This may be an issue as SCFA lower colonic pH thereby inhibiting pathogenic colonisation, and because butyrate provides a substrate for colonocytes and has anti-inflammatory properties(Reference Gibson, Probert and Van Loo26). In particular, the LFD restricts oligosaccharides, namely fructans and GOS that consist of fructose or galactose monomers (respectively) linked by glycosidic bonds that are not hydrolysed by human enzymes, and which when supplemented to the diet have been demonstrated to stimulate bifidobacteria numbers in the gut(Reference So, Whelan and Rossi27). Therefore, fructans and GOS are major sources of microbiota-accessible carbohydrate in the diet and restriction is likely to have a greater effect on those microbes that specialise in carbohydrate fermentation. Bifidobacteria in particular dedicate about 8 % of their total genome to carbohydrate metabolism, approximately 30 % more than most other gut microbiota(Reference Pokusaeva, Fitzgerald and van Sinderen28) and may explain why bifidobacteria are so affected by FODMAP restriction.
Several strategies have been investigated to mitigate against the impact of the LFD on the GI microbiota. A recent clinical trial of the LFD supplemented with a multi-strain probiotic (that included three strains of bifidobacteria) found the probiotic maintained absolute abundance of bifidobacteria during the LFD and therefore may be a useful adjunct to the LFD. Clinically, the number of patients that responded to the diet (29/51, 57 %) or the probiotic (30/53, 57 %) was greater than the number that responded to a sham diet (20/53, 38 %, P = 0⋅051) or placebo (19/51, 37 %, P = 0⋅048)(Reference Staudacher, Lomer and Farquharson16). Apart from flatulence, for which severity was reduced (P = 0⋅033), the probiotic did not significantly improve individual symptom severity compared with placebo, whereas the LFD reduced numerous symptoms including abdominal pain, bloating, flatulence and overall symptoms (Table 1). Therefore, it should be explained to patients that if they wish to take a probiotic with the LFD, depending upon the probiotic it may or may not improve symptoms but may prevent a reduction in bifidobacteria. Although probiotics would not maintain the native population of bifidobacteria, they may maintain overall numbers of bifidobacteria through exogenous supplementation(Reference Staudacher, Lomer and Farquharson16), and so could be useful in preventing the native bifidobacteria being at a competitive disadvantage once FODMAP are reintroduced. Probiotic co-supplementation is therefore one approach to the maintenance of bifidobacteria during the LFD; however, other benefits of fermentable fibre consumption, especially SCFA production and immune modulation, may not be affected by probiotic co-supplementation. One RCT reports the addition of a prebiotic β-GOS to the LFD, which although improved symptoms, it was not effective at maintaining luminal bifidobacteria or SCFA(Reference Wilson, Rossi and Kanno29).
Finally, it is possible that the detrimental effects of the LFD on the microbiota are reversed during the reintroduction and personalisation stage of the diet, but evidence of this is currently lacking in the literature and therefore long-term studies of the impact of all three stages of the LFD on both symptoms and gut microbiome are warranted. Until more is known about the effect of reintroduction, clinicians should address the effect on the microbiota when providing guidance on the LFD (Table 2).
Table 2. Clinical take-home messages to minimise and mitigate some of the challenges of the low FODMAP diet in clinical practice

Challenge 2: the low FODMAP diet may impact nutrient intake and diet quality
The LFD requires significant dietary changes for most patients. Foods are excluded from most of the commonly described food groups, including cereals/starchy foods (e.g. wheat-containing breads, pasta, pastries), fruit (e.g. apples, cherries, plums), vegetables (e.g. onion, cauliflower, celery) and dairy (many milk-containing products). Therefore, in theory, the nutrients most likely to be compromised during the LFD are calcium (restriction of lactose in dairy products), iron (restriction of iron-fortified breakfast cereals) and fibre (restriction of some grains, fruit and vegetables). Additionally, overall energy intake may be compromised, particularly considering the restriction of staple foods such as bread and pasta. However, the impact of the LFD on nutrient intake and dietary quality has not been extensively investigated.
Patients with IBS following LFD advice for 4 weeks were reported to have lower calcium intakes compared to patients following the habitual diet(Reference Staudacher, Lomer and Anderson11), and lower energy, carbohydrate and fibre intakes compared to patients following traditional IBS dietary advice (NICE diet)(Reference Böhn, Störsrud and Liljebo17). More recently, in ninety-five patients with IBS, calcium intake was not different between the LFD and sham diet groups at the end of the trial, although fewer patients in the LFD group at the end of the trial reached the calcium dietary reference value compared with baseline habitual diet indicating that calcium replacement should be addressed during LFD dietary counselling(Reference Staudacher, Ross and Briscoe30). More recently, a comparison of traditional IBS dietary advice (modified NICE diet) and LFD revealed both diets reduced overall energy intake, specifically a reduction in carbohydrate intake, but micronutrients, when correcting for the percentage of overall energy intake, were not affected with the exception of riboflavin(Reference Eswaran, Dolan and Ball31). However, when comparing within groups (i.e. comparing 4-week data to baseline data), the LFD but not ‘modified NICE diet’ significantly reduced the intake of some micronutrients including calcium, supporting earlier findings. Finally, one publication combined the results of two earlier RCT and reported low-fibre intakes in baseline habitual diet in 130 patients with IBS, with a mean intake of only 18 g/d and only six (5 %) achieving the dietary reference value for fibre intake (30 g/d)(Reference Staudacher, Ralph and Irving32). Macronutrient, calcium and fibre intakes following the LFD were not different compared to sham diet or habitual diet. However, the LFD marginally reduced diet quality, a measure of how closely the diet aligns with the World Health Organization dietary guidelines for the prevention of chronic disease, compared to control diets(Reference Huijbregts, Feskens and Räsänen33).
The impact of the LFD on vitamin intake is reported in two studies. In one report comparing the effect of a 4-week LFD on nutrient intake, a significant reduction in thiamine and riboflavin compared to baseline intake was shown, possibly due to the restriction of lactose-containing dairy products(Reference Eswaran, Dolan and Ball31), there was no effect on the intake of other vitamins. More recently, an assessment of 130 individuals randomised in two RCT to either a low FODMAP, habitual or sham diet for 4 weeks showed that vitamin B12 and selenium intakes were higher during the LFD compared to sham diet, and vitamin B12 was also higher during LFD compared to the habitual diet. Increases in intakes of these micronutrients may indicate a higher meat and potato consumption in view of some staple carbohydrates being restricted (e.g. bread, pasta, wheat-based cereal). Despite these differences in actual intake, there were no differences in the proportion meeting the dietary reference values for either vitamin B12 or selenium between the three diets(Reference Staudacher, Ralph and Irving32). Finally, there are currently no studies investigating the impact of the LFD on vitamin status (e.g. serum concentrations, etc.), and such investigations are required.
These data report the effect of stage 1 restriction of the LFD on nutrient intake and diet quality. However, the long-term effects of the LFD on nutrient intake were investigated in a study of patients 6–18 months after receiving FODMAP reintroduction advice(Reference O'keeffe, Jansen and Martin34). At the long-term follow-up, 82 % continued to follow an adapted FODMAP diet (i.e. stage 3 personalisation), while 18 % had returned to their habitual diet. In the adapted FODMAP group, total FODMAP intakes were significantly lower while nutrient intakes were similar to the habitual diet group. Another study demonstrated that fibre intake declined during stage 1 FODMAP restriction but returned to normal following FODMAP reintroduction and personalisation(Reference Harvie, Chisholm and Bisanz12). These findings suggest that FODMAP reintroduction and personalisation may resolve nutritional inadequacies that may occur with FODMAP restriction; however, both studies used a FFQ to estimate nutrient and FODMAP intakes, which may lack accuracy compared to prospective food records(Reference Bingham, Gill and Welch35,Reference Prentice, Mossavar-Rahmani and Huang36) . In general, clinicians should provide guidance on how to optimise nutrient intake and maintain diet quality while following the LFD (Table 2).
Challenge 3: the low FODMAP diet is complex to follow
The LFD is complex to follow for numerous reasons. First, the LFD restricts foods across a wide range of food groups, including starchy carbohydrate, fruit, vegetable and dairy foods as well as requiring vigilance for common food additives such as polyol sweeteners, fructose and inulin and the term ‘flavourings’ (that may include onion, garlic or other FODMAP-containing ingredients) and therefore can require extensive food knowledge and label reading. Secondly, there are numerous foods that contain minimal amounts of FODMAP and therefore allowed in small quantities but are disallowed in abundance. Thirdly, the diet is designed as a process of restriction and reintroduction rather than a long-term exclusion diet, and the reintroduction process can be confusing if unsupported. Fourthly, although the LFD per se has not been shown to impact the food-related quality of life, those with IBS who follow numerous different dietary restrictions may do so(Reference Guadagnoli, Mutlu and Doerfler37). For example, in the long-term, patients report that compared to their former habitual diet, the LFD is more costly, makes dining out at restaurants or friends/families houses more difficult, and makes travelling abroad more difficult(Reference O'keeffe, Jansen and Martin34).
Two RCT have shown that both LFD and NICE dietary advice were similarly effective in reducing overall symptoms(Reference Eswaran, Chey and Han-Markey18,Reference Bohn, Storsrud and Liljebo38) (Table 1). However, in one of these studies, the LFD was superior to NICE guidelines at reducing the severity of some individual symptoms, including abdominal pain, faecal urgency and bloating(Reference Eswaran, Chey and Han-Markey18) and resulted in greater improvements in the quality of life(Reference Eswaran, Chey and Jackson39). A more recent RCT comparing LFD and standard dietary advice (similar to NICE dietary advice) found LFD to be superior in the numbers achieving the primary outcome (≥30 % decrease in average daily worst abdominal pain) as well as a greater reduction in global IBS symptoms and post-prandial breath hydrogen production(Reference Patcharatrakul, Juntrapirat and Lakananurak20).
The NICE dietary advice for IBS include healthy eating, regular meal patterns, reducing fat, chilli and caffeine intake, but also include the elements of FODMAP restriction, including reducing onion, garlic and fruit intake, all of which ought to be considered when assessing the diet. Given NICE dietary advice is likely less restrictive and less complex than an LFD, guidelines have suggested this approach should be used first, and only if unsuccessful should an LFD be attempted. NICE dietary advice should always be considered as the first-line treatment for IBS dietary management; however, a trained dietitian should examine both overall diet quality, meal pattern and composition and FODMAP content during consultation and therefore a qualified decision can be made regarding which approach is most suitable for the individual patient (Table 2).
Challenge 4: patients require support to follow the low FODMAP diet effectively
Given the challenges of the LFD described, including the impact of the diet on the microbiome, nutrient intake, diet quality and its complexity, a registered dietitian with training in the delivery of the LFD should support patients in following the diet. Thus far, evidence for the successful implementation of the LFD has predominantly involved dietitian-led advice(Reference Staudacher, Lomer and Anderson11,Reference Halmos, Power and Shepherd13,Reference Staudacher, Lomer and Farquharson16,Reference de Roest, Dobbs and Chapman40) , although two studies have utilised nurse-led low FODMAP dietary advice(Reference Mazzawi, Hausken and Gundersen41,Reference Ostgaard, Hausken and Gundersen42) . Neither of the nurse-led studies were RCT and thus do not provide high-quality evidence for the effectiveness of this method of LFD delivery. In a retrospective analysis of patients with IBS advised to follow an LFD, education by a dietitian was associated with the greater achievement of dietary goals than non-dietitian education, in whom incomplete FODMAP restriction and unsuccessful reintroduction were reported(Reference Tuck, Reed and Muir43). Furthermore, some patients advised to follow the LFD by their general practitioner/family doctor or gastroenterologist and who are not referred for specialist advice from a dietitian have reported the advice received to be too simplistic, with simple checklists of ‘allowed’ and ‘disallowed foods’ or self-searching of the Internet used as methods of education(Reference Trott, Aziz and Rej44).
For these reasons, IBS guidelines specify that the LFD should preferably be delivered by a healthcare professional with expertise in the dietary management of IBS(4).
The need for dietitians to support patients in following the LFD has led to its own challenges in healthcare services delivery, including the need for adequate training in the diet and the demand for dietetic services. First, in terms of training, although dietary management of IBS is included in most pre-registration programmes in dietetics, critical understanding of the research evidence and the complexity of the different stages of the LFD require additional training. Post-registration training for dietitians can be provided by experienced colleagues and work shadowing and/or through completion of formal training courses provided by a wide range of educational providers across the world either face-to-face or through online learning. Secondly, in terms of the resulting high demand for dietetic services, group education is one approach that has been used in the UK National Health Service. In a non-randomised trial involving triaging patients to 1:1 advice or group education, both approaches were shown to be equally effective in terms of bloating, flatulence and faecal urgency, although 1:1 advice was superior for reducing abdominal pain(Reference Whigham, Joyce and Harper45). Therefore, group education, which relies on social interaction and peer support in addition to the expertise of the dietitian leading the group, may be an attractive option for healthcare providers as the cost per patient for dietetic intervention reduced from £139⋅20 to £67⋅19 using group education. In addition, there are many smartphone applications available to provide guidance on low FODMAP foods and these are widely used by dietitians(Reference Chen, Lieffers and Bauman46) and by patients to assist with following the LFD. However, the information provided on apps is unregulated and not all apps may provide the same information, leading to confusion for some patients. A small three-arm RCT in fifty-one people with IBS compared the efficacy of smartphone application v. dietitian advice v. a leaflet and showed that patients found the diet easier to implement when provided with guidance by a dietitian compared to other methods(Reference Dimidi, Whelan and Lomer47). However, patients reported similar levels of adequate relief of global IBS symptoms between dietitian advice (80 %) and using a smartphone app (63 %) and a similar reduction in symptoms from baseline measured using the IBS severity scoring system, although this may be a type II error due to study power. The provision of a leaflet alone was significantly less effective than 1:1 dietetic advice for symptom improvement indicating patients require more support than this(Reference Dimidi, Whelan and Lomer47). Larger, adequately powered clinical trials of optimal dietary delivery methods are required.
In conclusion, due to the complex nature of altering the whole diet, patients should be supported using the best available resource during this process to increase the likelihood of symptom improvement (Table 2).
Challenge 5: not all patients respond to the low FODMAP diet
Research data suggest that 50–80 % of patients will experience a positive symptom response to the LFD. Put another way, this means that 20–50 % of IBS patients do not respond symptomatically to the LFD despite having similar symptoms and demographics to those that do. The reason for non-response is under increasing investigation in order to optimise the selection of patients most likely to respond to the LFD, and to avoid unnecessary dietary restriction in those unlikely to respond. While adherence to the diet has been suggested as one reason for no response to the LFD(Reference McIntosh, Reed and Schneider15,Reference Schumann, Klose and Lauche22) , more recently, data have emerged suggesting measurable variation in both microbiota and faecal metabolites that differentiate those who go on to respond to the diet compared to those who do not.
A clinical trial randomised thirty-one patients with IBS to the LFD and thirty patients to NICE dietary advice, and stool samples were analysed at baseline using a commercially available GA-map dysbiosis test that uses fifty-four bacterial probes to identify ≥300 bacteria across different levels of phylogeny (Genetic Analysis AS, Oslo, Norway)(Reference Bennet, Bohn and Storsrud25). The nineteen (61 %) responders to the LFD (defined as a reduction of ≥50 points on the IBS-severity scoring system) had lower bacterial probe signal intensities for thirteen bacterial targets at baseline than the twelve (39 %) non-responders to LFD. Bacterial groups that were lower at baseline in responders were Bacteroides stercoris, Pseudomonas, Acinetobacter, Desulfitispora, Parabacteroides, Bacillus, Salmonella (Citrobacter, Cronobacter, Enterobacter), Corea, Ruminococcus gnavus, Clostridium, Firmicutes (Clostridia) and Streptococcus. Multivariate orthogonal partial least-squared discriminant analysis of bacterial profiles was able to reliably distinguish between responders and non-responders to the LFD at baseline (Q 2 = 0⋅54)(Reference Bennet, Bohn and Storsrud25).
In a further uncontrolled study, sixty-one adult patients with IBS followed the LFD for 4 weeks(Reference Valeur, Småstuen and Knudsen48), with the thirty-two (52 %) of responders (defined as a reduction of ≥50 points on the IBS-severity scoring system) had different baseline microbiota compared to the twenty-nine (48 %) non-responders. A group of five bacteria (Bacteroides fragilis, Acinetobacter, Ruminiclostridium, Streptococcus, and Eubacterium) were higher and five bacteria (Clostridia/Negativicutes/Bacilli, Actinomycetales, Anaerotruncus, Clostridiales and Shigella/Escherichia) were lower in responders than non-responders at baseline when measured using a dysbiosis test. From these ten bacteria, the authors developed a response index, and patients with a positive responder index score were five times more likely to have responded to the LFD (OR = 5⋅05, 95 % CI 1⋅58, 16⋅10) although this algorithm has yet to be tested prospectively.
While these results regarding microbiota differences from these two studies are promising, the findings differ between the studies despite both using the same intervention, similar selection criteria and test for assessing microbiota. There was only one common differentiating genus between the studies (Streptococcus); however, at baseline, it was lower in responders in one trial and higher in responders in the other, indicating there is still much to be discovered about how baseline microbiota may relate to symptom response to the LFD.
Some studies have examined faecal metabolites as a marker of bacterial activity in relation to predicting response to the LFD. An RCT in which forty-six adults with IBS were randomised to the LFD and forty-seven to a sham diet demonstrated that baseline faecal volatile organic compounds differed between the thirty-seven (80 %) LFD responders (defined as a reduction of ≥50 points on the IBS-severity scoring system) and nine (20 %) LFD non-responders with a high level of accuracy (97 %)(Reference Rossi, Aggio and Staudacher49). Preliminary data from a more recent study identified that the urine metabolome and faecal propionate may predict response to the LFD and confirmed that faecal volatile organic compound differs between responders and non-responders(Reference Wilson, Rossi and Kanno50). Further adequately powered studies are required to confirm these findings and identify the factors associated with response, or lack of response, to the LFD. Importantly, in the current absence of a widely-available predictive test, it is important to manage patients’ expectations when initiating the LFD (Table 2).
Discussion
Although there is now a wide range of evidence to support the use of the LFD in clinical practice for IBS, it is important to acknowledge the challenges with regards to long-term safety, implementation and likelihood of response to the diet. These issues should be raised and discussed with patients when advice on the LFD is provided.
Importantly, all health professionals should acknowledge the challenges of the LFD discussed here and provide guidance on where to access reliable information on the diet. Unguided use of the LFD may lead to unnecessary long-term restriction and may contribute to nutritional deficiencies and exacerbate food phobia, especially when patients are later encouraged to reintroduce foods. In addition, the LFD may be less likely to be effective when not explained with sufficient detail as subtleties could be missed or inaccurate information about the foods to restrict could be provided. This could lead to patients feeling they have tried the diet and it did not work, whereas it may have been effective if sufficient guidance was provided and if such advice was followed correctly. It should be acknowledged that an appointment to undertake the full assessment of individual symptoms, bowel habit, current diet and to provide dietary counselling on a whole dietary change should last approximately 45–60 min(Reference Whelan, Martin and Staudacher5). Tailored advice on how to optimise the quality of the current diet and suitable swaps to make the diet low in FODMAP as well as nutritionally adequate should be provided. As the LFD restricts food with both laxative and gas-forming potential(Reference Murray, Wilkinson-Smith and Hoad7), it should be used with caution in patients with constipation (either IBS-C or IBS-M) and additional advice on maintaining suitable fibre and fluid intake should always be provided.
To date, the research evidence for using the LFD in IBS is supportive of a clinical benefit on symptoms and is now included as the second-line dietary approach (after NICE dietary advice) in some national guidelines(4,Reference McKenzie, Bowyer and Leach51) . However, all RCT of the diet have been delivered using advice from a registered dietitian and evidence for the effectiveness of the diet when provided in less structured formats or by other health professionals is limited. The training and clinical expertise of dietitians mean they are well-placed to assess habitual diets and advise on the dietary changes most likely to benefit patients. There is not a one size fits all approach and advice may include a combination of fibre, probiotics and other dietary and lifestyle approaches including the LFD. Therefore, wherever possible, assessment and dietary counselling should be provided by health professionals with expertise in diet and gastroenterology.
Conclusion
When provided by an adequately trained health professional, the LFD is a clinically effective treatment option for IBS. Challenges of the LFD should be acknowledged and discussed with the patient during the first consultation, and where possible referral to an appropriately trained dietitian should be made.
Financial Support
None.
Conflicts of Interest
B. W. undertook a doctoral fellowship funded by Clasado Biosciences Ltd. and is currently undertaking a research programme funded by the Medical Research Council and Danone. S. R. C. undertook a doctoral fellowship funded by Kenneth Rainin Foundation and is currently undertaking a research programme funded by The Leona M and Harry B Helmsley Charitable Trust. K. W. is currently or has recently been in receipt of research funding from a range of government bodies including the Medical Research Council, National Institute of Health Research, charitable bodies including Crohn's and Colitis UK, ForCrohns, The Leona M and Harry B Helmsley Charitable Trust and Kenneth Rainin Foundation, and industry bodies including the Almond Board of California, Clasado Biosciences Ltd., Danone, International Nut and Dried Fruit Council and Nestle. K. W. is the co-inventor of an app to assist people to follow the low FODMAP diet (FoodMaestro). None of these funding bodies contributed to the content or writing of this manuscript.
Authorship
K. W. and B. W. devised the outline for the manuscript. All authors wrote the manuscript.





