- MDI
Mental Development Index
- PN
parenteral nutrition
Aluminium is the most common metallic element and the third most common element after oxygen and silicon. Due to its reactivity, aluminium exists mostly in the form of ores, and free aluminium is rarely found. Historically, aluminium was regarded as more precious than gold or silver; Napoleon III was said to have served his most honoured guests from aluminium plates while less important visitors ate from gold platters. Aluminium is ubiquitous, but has no known biological role. Although lifetime exposure to aluminium is high, this does not pose problems for healthy individuals with normal renal function. However, aluminium accumulates in the body when protective gastrointestinal mechanisms are bypassed, renal function is impaired, or exposure is high; all of these situations are found frequently in sick preterm infants who are receiving parenteral nutrition (PN)(Reference Bishop, Morley and Day1).
Health effects of aluminium exposure
Clinical manifestations of aluminium toxicity have been recognised for many years, and include dementia, bone disease and anaemia. These problems were initially identified in patients with renal impairment exposed to high concentrations of aluminium from dialysis solutions and phosphate binders, who developed so-called ‘dialysis dementia’. Up to 80% of patients with dialysis dementia exhibit motor impairment, with myoclonic jerks, ataxia and dyspraxia(Reference Burn and Bates2). Cortical atrophy of the frontal lobes has been reported on brain scans(Reference Cusamo and Savazzi3) and autopsy studies of patients have shown particularly high concentrations of aluminium in grey matter(Reference McDermott, Smith and Ward4). Bolla et al. (Reference Bolla, Briefel and Spector5) performed detailed neurocognitive testing in adults undergoing dialysis, and reported that serum aluminium concentrations were predictive of visual memory. Associations between aluminium concentrations and tests of frontal lobe function and attention/concentration were also seen, although only in subjects with lower vocabulary scores. Similar neurological and cognitive problems were subsequently identified in adults receiving PN solutions, and also in aluminium smelting plant workers, especially those exposed before the new smoke hoods were introduced in 1972(Reference White, Longstreth and Rosenstock6).
Adverse effects of aluminium on bone have been identified in adults with uraemia and low turnover osteomalacia(Reference Ott, Maloney and Klein7) and in those with normal renal function undergoing long-tern PN(Reference Ott, Maloney and Coburn8). Both groups of patients had low bone formation on iliac crest biopsy, with patchy osteomalacia. Histochemical staining of biopsy samples showed aluminium accumulation at the mineralisation front, which was quantified as surface-stainable aluminium. The latter correlated closely with quantitative measurements of aluminium in bone determined by atomic absorption spectroscopy. These findings were supported by multiple studies in several species of experimental animals including rats(Reference Goodman, Gilligan and Horst9), piglets(Reference Sedman, Alfrey and Miller10) and dogs(Reference Goodman, Henry and Horst11).
Aluminium exposure in preterm infants
Moreno et al. (Reference Moreno, Dominguez and Ballabriga12) calculated that PN solutions were the main source of aluminium exposure in neonates, accounting for 89% of total aluminium intake. In earlier PN solutions (prior to the mid-1990s), aluminium contamination occurred mostly from casein hydrolysates and trace element components, but the problem with more modern PN solutions relates mainly to small-volume acidic solutions stored in glass vials, notably calcium gluconate which was found to account for 81% of the contamination in one study(Reference Mouser, Wu and Herson13). It has been recognised for some time that, when fed parenterally, infants retain >75% of the aluminium (compared to approximately 40% in adults), with high serum, urine and tissue levels(Reference Moreno, Dominguez and Ballabriga12, Reference McGraw, Bishop and Jameson14). For example, Sedman et al. (Reference Sedman, Klein and Merritt15) prospectively studied plasma and urinary aluminium concentrations in eighteen premature infants receiving intravenous therapy and in eight term infants receiving no intravenous therapy. They also measured bone aluminium concentrations in autopsy specimens from twenty-three infants, including six who had received at least 3 weeks of intravenous therapy. Preterm infants who received intravenous therapy had high plasma and urinary aluminium concentrations compared with normal controls. The bone aluminium concentration was also ten times higher in infants who had received at least 3 weeks of intravenous therapy than in those who had received limited intravenous therapy.
Although aluminium exposure and tissue aluminium accumulation were well documented in neonates two decades ago, it was unclear at the time whether this exposure had any health consequences. A causal relationship between early aluminium exposure and adverse health outcomes cannot be established in observational studies, particularly in preterm infants in whom the duration of PN (and hence, aluminium exposure) is very likely to act as a proxy for poor health, which is itself associated with adverse outcome. Thus, an experimental study was required, with preterm infants randomised to different aluminium exposure and follow-up to measure health outcomes. While it was not ethical to randomise a group of infants to receive ‘high’ aluminium exposure, it was ethical and feasible to randomise them to receive a lower aluminium exposure than they would receive in normal clinical practice.
Randomised trial of aluminium exposure from parenteral nutrition in preterm infants
To investigate the short- and long-term health effects of neonatal intravenous aluminium exposure, a randomised double-blind trial was initiated in 1988, comparing standard PN solutions with solutions specially sourced for low aluminium content. Details of the original trial design are reported elsewhere but summarised briefly here(Reference Bishop, Morley and Day1). Two hundred and twenty-seven preterm infants with birthweight <1850 g were recruited from a neonatal unit in Cambridge, UK. Infants were eligible if a clinical decision was made to start PN, and were randomly assigned to receive either standard PN solution or a specially sourced low-aluminium solution. Details of the composition of the two solutions are shown in Table 1, together with the measured aluminium concentrations in each solution. The solutions were identical except that the aluminium-depleted solution contained calcium chloride instead of calcium gluconate. The use of a mixed sodium–potassium phosphate solution in place of potassium acid phosphate minimised the increase in chloride. By design, the total aluminium intake when the infant received 180 ml/kg per d differed markedly; 45 μg/kg per d for the standard solution compared with only 4–5 μg/kg per d for the aluminium-depleted solution. All decisions on infant feeding were made by the clinicians responsible for the care of the infant; the study team were not involved in this aspect. Data were collected on the clinical course of each infant, detailed records of exact intravenous and oral intake, daily blood samples for electrolytes, calcium and acid-base status, and weekly samples for plasma chloride.
Table 1. Composition and aluminium content of the standard and aluminium-depleted intravenous feeding solutions used in a randomised trial(Reference Sedman, Klein and Merritt15)
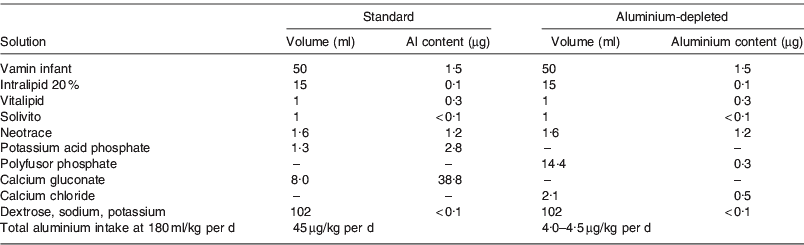
Vamin infant contained essential amino acids without added electrolytes. Intralipid 20% was a fat emulsion containing 20 g fatty acids/dl. Vitalipid contained fat-soluble vitamins and Solivito contained water-soluble vitamins. Neotrace was an in-house preparation containing Cu and Zn only. Vamin infant, intralipid 20%, vitalipid and solivito were manufactured by Kabi Vitrum.
Cognitive outcome at 18 months post-term(Reference Bishop, Morley and Day1)
At 18 months corrected age, all surviving infants were invited for a follow-up examination. A single investigator, blind to the PN allocation, assessed cognitive development using the Mental Scale of the Bayley Scales of Infant development(Reference Bayley16), from which the Mental Development Index (MDI) was derived. The number of days of intravenous feeding for infants tested at 18 months did not differ by randomised group. Overall, there was no difference in MDI between randomised groups and no difference in the proportion of infants considered to have neuromotor impairment. However, preterm infants exposed for >10 d to standard solutions had impaired neurologic development at 18 months, and were significantly more likely to have MDI<85 (n 41 for aluminium-depleted group and n 39 for standard group), placing them at increased risk for subsequent educational problems. For the 157 infants without neuromotor impairment, increasing neonatal aluminium exposure was associated with a reduction in the Bayley MDI, with an adjusted loss of one point per day of intravenous feeding with the standard solution. These findings strongly suggested that prolonged exposure to PN solutions that are routinely contaminated with aluminium might have lasting adverse consequences for cognitive outcome in this vulnerable group.
Follow-up at 13–15 years
To test the hypothesis that neonatal aluminium exposure would have persisting adverse effects on cognitive outcome during adolescence, and adverse effects on bone health, fifty-nine subjects from the original cohort (26% of those randomised; 32% of survivors; 33% of those eligible for follow-up) were invited for follow-up at age 13–15 years. Subjects with neuromotor impairment or with Bayley MDI<85 at 18-month follow-up were excluded, because children with an existing impairment would be unable to complete the cognitive tests in the follow-up protocol. A detailed battery of tests was administered, evaluating overall cognitive level (intelligence quotient (IQ))(Reference Wechsler17) and also specific functions hypothesised to be potentially affected by early aluminium exposure, including tests of academic attainment(Reference Wechsler18, Reference Wechsler19), different aspects of memory functions(Reference Talley20–Reference Corsi22), and higher level functions such as planning and organising behaviour(Reference Emslie, Wilson and Burden23). Bone mass was measured using dual X-ray absorptiometry at the lumbar spine, hip and whole body. Fifty-nine subjects were seen for follow-up at age 13–15 years; thirty-three from the aluminium-depleted group and twenty-six from the standard group. Compared with subjects not seen, those who were followed up had significantly higher birthweight SDS, suggesting they were a ‘lower risk’ group in terms of later adverse outcomes. The total duration of intravenous feeding for subjects followed up was not significantly different from randomised groups (12·5 (sd 8·8) days for aluminium-depleted v. 13·2 (sd 9·2) for controls, P=0·8). However, as expected, mean, median (25th, 75th centiles), minimum and maximum exposure in the two groups were significantly different (3·0 (sd 0·8), 28 (17, 46), 4, 152 μg/kg for the aluminium-depleted group and 21·3 (sd 7·2), 280 (91, 417), 19, 840 μg/kg for the control group (P<0·001 for all)).
Cognitive outcome
As at 18 months of age, no significant differences in cognitive outcome were found between randomised groups during adolescence. Non-randomised analyses were also performed to assess the impact of aluminium exposure, because an infant's actual exposure depended not only on the PN solution received but also on the duration of intravenous feeding, which varied considerably. The actual neonatal exposure to aluminium (shown in Fig. 1) covered a wide range, with considerable overlap between randomised groups. No significant differences were found when comparing subjects with total neonatal aluminium exposure above or below the median (55 μg/kg).

Fig. 1. Neonatal aluminium exposure for subjects studied at 13–15 years, according to randomised parenteral nutrition (PN) solution in a randomised trial(Reference Sedman, Klein and Merritt15).
These findings must be considered in the context of the fact that the subjects seen at 13–15 years were a selected group, excluding those with known neuromotor impairment at 18 months or a Bayley MDI<85; they also had higher birthweight SDS than subjects who were not followed. Hence, it could be argued that the findings show no evidence of longer-term cognitive effects in this relatively lower risk cohort who had normal cognitive outcome at 18 months post-term, but cannot perhaps be generalised to smaller infants who already have evidence of neurocognitive impairment apparent in infancy: in effect, the follow-up protocol may have excluded the children already adversely affected by neonatal aluminium exposure. However, the sub-group of subjects studied at 13–15 years (particularly those subjects who had received more than 10 d of PN) showed the same trend towards higher Bayley MDI at 18 months in the aluminium-depleted group as observed in the larger cohort. Statistical significance was not reached for this comparison, possibly due to the small sample sizes available for the analyses; nevertheless these data suggest that the children followed-up were fairly representative of all subjects seen at 18 months in terms of the effects of aluminium exposure on cognitive outcome. One possibility is that those with Bayley MDI>85 in infancy were able to compensate subsequently for any adverse effect of early aluminium exposure.
Bone outcomes(Reference Fewtrell, Bishop and Edmonds24)
Subjects randomised to the aluminium-depleted PN solution during the neonatal period had significantly higher lumbar spine bone mineral content and bone area at age 13–15 years, apparently reflecting larger bones with a concomitant increase in bone mineral. In non-randomised analyses, aluminium exposure as a continuous variable was not associated with later bone mass. However, there was evidence of a threshold effect. Subjects with neonatal aluminium exposure above the median (55 μg/kg) had significantly lower hip bone mass, independent of their bone or body size. This effect was not seen at the lumbar spine or for whole body bone mass.
The mechanism for the observed effects of early aluminium exposure on later bone health is unclear. A direct effect on bone structure is unlikely since the skeleton will have remodelled more than once in the intervening years. It is possible that aluminium modifies the response of bone cells to external stimuli such as subsequent loading from physical activity or nutritional exposure. This could also explain the apparent site-specificity of effects, with effects on lumbar spine bone mass in the randomised comparison, but a threshold effect observed on hip bone mass. It is well recognised that interventions may have differential effects at different skeletal sites. For example, exercise interventions typically affect only the loaded bones(Reference Fewtrell, Lucas and Sampson25), while leptin has been shown to have different effects on the trabecular and appendicular skeleton, possibly related to differential effects on cortical and trabecular bone(Reference Cirmanova, Beyer and Starka26). An alternative explanation is that bone effects are another manifestation of aluminium neurotoxicity; it is now recognised that bone remodelling is partly under the control of the central nervous system(Reference Elefteriou27). In animals, a number of neuropeptides affect bone formation via the hypothalamus, with signal transmission to bone cells via the sympathetic nervous system. If this is the mechanism, the observed adverse effects on bone may represent another facet of neurotoxicity.
Study limitations
The main limitation of the most recent follow-up study was the attrition rate, with only 30% of the original cohort seen at 13–15 years. This limits the power of the follow-up study, allowing detection of a difference of approximately 0·7 sd between randomised groups at 5% significance. In the event, this may not have been an issue, because the effect size for lumbar spine was of this magnitude. Subjects seen for follow-up also had significantly higher birthweight sd scores than those not seen, and one would suppose that any effect of aluminium seen in the follow-up study might be greater in more vulnerable, smaller infants who were not studied. The follow-up study also excluded subjects already identified as having abnormal development at age 18 months, who would be regarded as more vulnerable and potentially at greater risk of adverse effects on cognitive outcome or bone health. It may therefore have underestimated any effect of aluminium exposure.
Interpretation and practical implications of the findings
Data from this clinical trial suggest that neonatal aluminium exposure from PN in the high-risk preterm infant may have adverse effects on later bone health, as well as short-term cognitive outcome. Although there was no strong evidence for effects on later cognitive outcome, the group of subjects followed at 13–15 years were a selected population with normal development at 18 months; it is unclear whether persistent or additional adverse effects would be apparent in the subjects who already demonstrated sub-optimal development at 18 months of age. The observed effects are plausible given the known toxicity of aluminium for brain and bone seen in adults and in animal models. This is the only experimental study to systematically examine the health effects of aluminium in any population with high exposure and, despite its limitations, it seems unlikely that it will be repeated.
It is important to consider the likely practical significance of the observed effect of aluminium exposure on bone mass. This is difficult to quantify since there are no data directly relating bone mass at age 13–15 years to later fracture risk. Hip bone mass was 7·6% lower in subjects with neonatal aluminium exposure above the median, while the difference in lumbar spine bone mineral content was approximately 0·7 sd between groups (representing approximately 14% of population variance assuming a normal distribution), and the difference in lumbar spine bone mineral density was 0·36 sd (representing approximately 7% of population variation). These figures can be considered in the context of the study of Hernandez(Reference Hernandez28), who estimated that the peak bone mass was a better predicter of osteoporosis risk than either the age at menopause or the rate of age-related bone loss later in life, and calculated that a 10% increase in peak bone mass would delay the onset of osteoporosis by 10 years. Given the sizeable number of contemporary infants undergoing intensive care and still exposed to aluminium via PN, these findings have contemporary relevance. PN is also used more aggressively in modern neonatal units, starting earlier and with more rapid advancement than was typical at the time of the clinical trial; hence neonatal aluminium exposure may be greater. Furthermore, recommended mineral intakes for preterm infants are now higher than what it was 20 years ago, so preterm infants are exposed to greater volumes of calcium gluconate, the main offender in terms of aluminium intake.
Regulatory aspects of aluminium exposure
Potential methods for lowering aluminium exposure from PN solutions are given in Table 2. Despite widespread recognition of the problem, until recently little progress had been made on reducing exposure. Following a review of the literature, the Food and Drug Administration recommended that daily aluminium intakes should not exceed 5 μg/kg per d in vulnerable patients(29), including preterm infants. Manufacturers were required to ensure that large volume parenterals do not contain more than 25 μg/l of aluminium and to label them as such. No restrictions were placed on the aluminium content of small volume parenterals, but manufacturers were required to label them with the estimated aluminium content at expiry. While this represented an advance, Poole et al. (Reference Poole, Hintz and Mackenzie30) calculated that meeting the Food and Drug Administration recommendations was currently impossible in patients <50 kg using available products. Furthermore, calculated aluminium intake in patients <3 kg in their study was 30–60 μg/kg per d – higher than in the randomised trial discussed in this paper. In a more recent study, the same investigators measured the actual aluminium content of PN solutions being administered to forty preterm infants and found that intakes were still three to five times the recommended Food and Drug Administration limit, although significantly less than the intake calculated using manufacturers’ values on product labels(Reference Poole, Schiff and Hintz31). Most recently (2010), following a review of available data, the UK Medicines and Healthcare Regulatory Authority recommended that calcium gluconate in small volume glass containers should not be used for repeated or prolonged treatment in children <18 years, including preparation of PN(32). Manufacturers should now be required to address the issue at least for this particular component of PN solutions, and this represents an important step towards addressing the problem of aluminium exposure and toxicity in vulnerable infants and children.
Table 2. How can neonatal aluminium exposure from parenteral nutrition (PN) solutions be minimised using currently available solutions? Potential methods and barriers to implementation

Acknowledgements
This study was supported by a research grant from UK Medical Research Council. The authors declare no conflicts of interest with respect to this paper. M. F. wrote the manuscript and was co-principal investigator for the follow-up study of the clinical trial, responsible for the design, analysis and interpretation of bone outcomes. C. E. was co-principal investigator for the follow-up study, responsible for the design and analysis of cognitive outcomes and contributed to the drafting and revision of this paper. E. I. contributed to the design of the cognitive parts of the follow-up study, analysis of cognitive data and drafting of this manuscript. N. B. was designer and principal investigator for the original randomised controlled trial and contributed to the drafting of this paper. A. L. was co-designer of the original randomised controlled trial and contributed to the drafting of this paper.





