Background
Diabetes is a heterogeneous set of disorders characterized by glucoregulatory abnormalities. Worldwide prevalence of diabetes is 9.8% in men and 9.3% in women (Danaei et al., Reference Danaei, Finucane, Lu, Singh, Cowan, Paciorek, Lin, Farzadfar, Khang, Stevens and Rao2011). In New Zealand, the overall prevalence of diabetes is approximately 7% and this is higher in men than women (8.3% vs 5.8%), and in Māori (7.4%) as compared to NZ Europeans (5%) (Coppell et al., Reference Coppell, Mann, Williams, Jo, Drury, Miller and Parnell2013). Importantly, type 2 diabetes mellitus (T2DM) is associated with an increased risk of incident and recurrent depression, with evidence suggesting a bidirectional association between the two (Pan et al., Reference Pan, Lucas, Sun, van Dam, Franco, Manson, Willett, Ascherio and Hu2010). In New Zealand, up to one quarter of patients with T2DM are currently taking an antidepressant medication, with use more likely in women, New Zealand European, obese patients and those receiving multiple medications. (Chepulis et al., Reference Chepulis, Morison, Lao, Keenan, Paul and Lawrenson2020).
While medication use for depression is relatively uncommon in T2DM, less is known about the prevalence of the condition. International data suggested that at least half of people with T2DM suffer with depression (Sartorius, Reference Sartorius2022), and New Zealand reports on the strong association between mental health and chronic disease (Scott et al., Reference Scott, Oakley Browne, McGee and Elisabeth Wells2006). However, it has been suggested that a large proportion of depression goes undiagnosed in this population, either due to clinical inertia or a range of patient factors (Sartorius, Reference Sartorius2022).
The importance of managing depression in diabetes patients should not be understated, though comorbidity of T2DM and depression can interfere with and limit the effectiveness of treatment for diabetes. Antidepressant medications may have a direct effect on glycemic control that are independent of its effect on weight and mood (Khapre et al., Reference Khapre, Kant, Sharma and Sharma2020), and consequences of depression in diabetic patients can be chronic and severe: the presence of depression in a person with diabetes may lead to 36.8% increase in coronary artery disease and a 47.9% increase in cardiovascular mortality (Farooqi et al., Reference Farooqi, Khunti, Abner, Gillies, Morriss and Seidu2019).
An integrated approach involving treatment of both the mental health condition and diabetes has been shown to be the best approach for improving outcomes in primary care, including improved HBA1c levels and remission of depression symptoms (Sartorius, Reference Sartorius2022). However, for clinicians to be able to appropriately manage all relevant conditions, they must be first identified. Thus, this small study aims to explore the efficacy of using a Patient Health Questionnaire – 9 (PHQ-9) tool for screening for depression in people with diabetes.
Methods
For this pilot study, a cross-sectional study method was used: 100 consecutive patients with T2DM who presented for repeat medications and/or annual diabetic review at one of two specific medical clinics in Hamilton were screened for depression using the PHQ-9, a valid and reliable screening tool for detecting depression (Atlantis et al., Reference Atlantis, Joshy, Williams and Simmons2017). The population at these clinics is a mix of high need patients including pacific and Māori (total registered patients around 13 000). Patients were included if they were aged 18–85 years and were at least 1-year post-T2DM diagnosis.
Data were collected from April to October 2022, either by face to face or phone consultations (during COVID lockdowns). Other data including BMI (height and weight), age, sex, ethnicity, HBA1c level, current use of antidepressant, current diabetes and cardiovascular medications and duration of diabetes were collected from the practice electronic patient management system. The data were collected by the primary author, a general practitioner at one of these clinics and his trainee intern. Depression was scored as mild (5–9), moderate (10–14) or severe (above 14) (Manea et al., Reference Manea, Gilbody and McMillan2015). This study got Ethics Approval from Health and Disability Ethics Committee (HDEC), New Zealand with approval number RM8309 https://ethics.health.govt.nz/.
Results
The demographics of the study population is given in Table 1. The mean age of patients was 59 years (range 18–85 years), and 59% of participants were male. Twenty nine percent of patients were New Zealand European, 9% were Māori with the remainder being of Pacific (16%), Asian (15%) and Other (31%) descent. The mean time since diabetes diagnosis was 10 years (Table 1).
Table 1. Study population demographics

Using the PHQ-9 tool, 18 patients were found to have a score of ≥ 5, of which two were currently prescribed a selective serotonin reception inhibitor (SSRI). A further 11 patients had a score of <5 but were active users of antidepressant medication (six were using SSRIs, four were prescribed other antidepressants such as venlafaxine and one patient was prescribed benzodiazepines). Overall, the prevalence of depression (known and previously unknown) was 29/100 (29%). Of the 18 previously undiagnosed patients, 8 had moderate to severe depression (requiring urgent active clinical management of depression) and 10 had mild depression.
By ethnicity, total and previously undetected prevalence was 41.3%/24.1% for European patients, 33.0%/33.0% for Māori patients, 25.0%/25.0% for Pacific patients, 13.3%/6.7% for Asian and 21.7%/6.5% of those of “Other” ethnicities.
Overall, 67 out of 100 patients were prescribed angiotensin-converting enzyme inhibitors/angiotensin receptor blockers with 25 patients regularly prescribed other forms of anti-hypertensive medications. Similarly, 68% patients were prescribed statin medications.
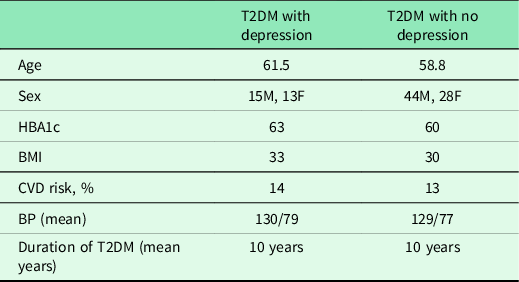
In our small cohort, there were no obvious differences between the demographics of those T2DM patients with and without depression with regard to age, sex, HBA1c, Cardio Vascular Disease (CVD) risks, blood pressure or duration of diabetes (Table 2).
Table 2. T2DM patient descriptors for those with and without depression
Discussion
Previously, undetected depression was common in our small cohort of T2DM patients, and the PHQ-9 tool was shown to be an easy-to-administer and effective screening tool in this group. Although our pilot study group was not collected in a way to be representative of the local demographic or the general practice population, it did identify that a large proportion of Māori, European and other patients were potentially being missed for treatment or intervention of depression. This aligns with other research that suggests that depression is often under-diagnosed or under-treated in diabetes (Sartorius, Reference Sartorius2022), including in New Zealand Māori (Atlantis et al., Reference Atlantis, Joshy, Williams and Simmons2017; McClintock et al., Reference McClintock, Blackmore, Chepulis, Fraser and Paul2022).
While our study is small, it does indicate that depression affects up to 30%–40% of patients with T2DM, which is comparable to that reported elsewhere (Egede & Ellis, Reference Egede and Ellis2010; Alhunayni et al., Reference Alhunayni, Mohamed and Hammad2020). We suggest that while depression is recommended to be a routine part of diabetes screening (The Annual Diabetes Review, 2022), it should be fully integrated into the annual diabetic review including the portal forms. We also suggest that it should be routinely used for opportunistic screening tool for patients coming for repeat prescriptions/follow-up, noting that effective communication between health professionals and patients is required to tease out additional concerns during these routine visits (Dowell et al., Reference Dowell, Stubbe, Macdonald, Tester, Gray, Vernall, Kenealy, Sheridan, Docherty, Hall and Raphael2018).
This was a small cross-sectional study localized to only two clinics in Hamilton which may not represent the wider Waikato region or diverse New Zealand population. Our data do suggest, however, that a larger, multicenter study is required to explore the prevalence of undiagnosed depression in patients with T2DM, particularly in those from different ethnic groups. Future studies should also include a comprehensive review of patients with mild depression who may be being managed without medication as this was not evaluated in our cohort. Further, it would be interesting to correlate these data to medication compliance and prescribing data, as Māori and Pacific people often seek help late and/or are under-prescribed mental health medications (Farooqi et al., Reference Farooqi, Khunti, Abner, Gillies, Morriss and Seidu2019).
Lastly, we note that depression is rarely seen in primary care in isolation and it often presents alongside anxiety and other conditions. Thus, while the PHQ-9 tool has been demonstrated to be effective at identifying patients with undetected depression, it may be more useful to use this alongside other tools such as the GAD-7 for anxiety and or the generic Kessler-10 (Vasiliadis et al., Reference Vasiliadis, Chudzinski, Gontijo-Guerra and Préville2015). These tools should be evaluated in further studies.
Conclusion
PHQ-9 scoring is an easy-to-administer tool to screen for depression in patients with diabetes, with data suggesting that approximately a quarter of patients may be undiagnosed. We suggest that PHQ-9 screening should be an integral part of the annual diabetic review for early detection and management of depression in patients with diabetes.
A limitation of this pilot study is that this study design may have missed patients with mild depression that was being managed without medication; however, these should be included in future studies.
Acknowledgements
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines (Health and Disability Ethics Committee (HDEC) New Zealand, with approval number RM8309).
Authors’ contribution
Data used to generate the results are available on request. Please contact corresponding author.
Financial support
This research did not receive any specific funding.
Competing interests
None.





