Introduction
Neonatal abstinence syndrome (NAS) is a multi-system disorder observed in infants experiencing withdrawal from opioid exposure in utero (Stover and Davis, Reference Stover and Davis2015; McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016). Care of infants typically begins with non-pharmacologic comfort measures to decrease the severity of symptoms and mitigate negative neonatal outcomes (Maguire, Reference Maguire2014; Edwards and Brown, Reference Edwards and Brown2016). However for infants who do not respond, pharmacotherapy may be warranted to manage the symptoms of withdrawal (McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016). Traditionally, pharmacologic treatment for infants with NAS is completed in-hospital until infants are stable and fully weaned from medication. However, there is some evidence to suggest that some hospitals may continue an outpatient weaning regimen (Napolitano et al., Reference Napolitano, Theophilopoulos, Seng and Calhoun2013; Chau et al., Reference Chau, Nguyen, Miladinovic, Lilly, Ashmeade and Balakrishnan2016), as many infants with NAS are otherwise healthy (Burns et al., Reference Burns, Mattick, Lim and Wallace2007; O’Grady et al., Reference O’Grady, Hopewell, White, O’Grady, Hopewell and White2009). This type of combined inpatient/outpatient treatment may assist to alleviate the strain of NAS on the healthcare system and decrease separation of mothers and infants. However, there is currently no consensus or standard of care to guide outpatient weaning (Chau et al., Reference Chau, Nguyen, Miladinovic, Lilly, Ashmeade and Balakrishnan2016; Patrick et al., Reference Patrick, Schumacher, Horbar, Buus-Frank, Edwards, Morrow, Ferrelli, Picarillo and Soll2016). Understanding the evidence regarding outpatient weaning for NAS is an important consideration for organizations seeking to adopt this method. To date, there are no systematic reviews available on outpatient weaning for NAS. Thus, the purpose of this systematic review was to assess the existing literature regarding the effectiveness and safety of outpatient pharmacologic weaning on NAS outcomes for infants with NAS. The specific review question was: Among infants with NAS who require pharmacologic treatment, what is the effect of a combined inpatient/outpatient weaning (eg, home weaning) versus in-hospital weaning on NAS outcomes (eg, length of stay, duration of treatment, cost, breastfeeding) and infant safety.
NAS presents as central nervous system hyperirritability, autonomic nervous system dysfunction, and gastrointestinal disturbances (Kocherlakota, Reference Kocherlakota2014; Stover and Davis, Reference Stover and Davis2015). Defining characteristics of the syndrome include a high pitched cry, irritability, fever, tremors, excessive sucking, weight loss, loose stools, poor feeding, sleep disturbances, excoriation of the skin, respiratory distress, and seizures (Finnegan et al., Reference Finnegan, Connaughton, Kron and Emich1975). Clinical manifestations of the syndrome are variable and are based on the type of opioid, timing of exposure, and maternal and placental metabolism (Hudak and Tan, Reference Hudak and Tan2012). Initial signs of NAS are often observed within the first 24–72 h (Hudak and Tan, Reference Hudak and Tan2012; Kaltenbach and Jones, Reference Kaltenbach and Jones2016); with most infants demonstrating signs of withdrawal within 12 h of birth (Kaltenbach and Jones, Reference Kaltenbach and Jones2016).
The incidence of NAS has risen dramatically in the United States. Between the years 2000 and 2009 the incidence of NAS increased from 1.20 (95% CI: 1.04–1.37) to 3.39 (95% CI: 3.12–3.67) infants per 1000 hospital births annually (Patrick et al., Reference Patrick, Schumacher, Horbar, Buus-Frank, Edwards, Morrow, Ferrelli, Picarillo and Soll2016). In 2013, a total of 4% of neonatal intensive care unit (NICU) admissions across the United States were attributed to a diagnosis of NAS (Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015). Similar increases in the incidence of NAS have been reported in Canada (Davies et al., Reference Davies, Gilbert, Johnson, Petersen, Nazareth, O’Donnell, Guttmann and Gonzalez-Izquierdo2016) and across Western Australia (O’Donnell et al., Reference O’Donnell, Nassar, Leonard, Hagan, Mathews, Patterson and Stanley2009), indicating the impact from a global perspective. The higher rates of NAS correspond to the increased incidence of maternal opioid use during pregnancy (Epstein et al., Reference Epstein, Bobo, Martin, Morrow, Wang, Chandrasekhar and Cooper2013; Krans et al., Reference Krans, Cochran and Bogen2015), inclusive of illicit opioid use (Cicero et al., Reference Cicero, Ellis and Harney2015), prescribed opioids for pain (Ailes et al., Reference Ailes, Dawson, Lind, Gilboa, Frey, Broussard and Honein2015; Warren et al., Reference Warren, Miller, Traylor, Bauer and Patrick2015), and the rise in opioid replacement therapies for pregnant women with addictions (Jansson et al., Reference Jansson, Velez and Harrow2009; O’Grady et al., Reference O’Grady, Hopewell, White, O’Grady, Hopewell and White2009).
Numerous negative outcomes have been associated with NAS including admission to a special care nursery (Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015; Uebel et al., Reference Uebel, Wright, Burns, Hilder, Bajuk, Breen, Abdel-Latif, Feller, Falconer, Clews, Eastwood and Oei2015), a lengthy hospitalization period (Wachman et al., Reference Wachman, Newby, Vreeland, Byun, Bonganzi, Bauchner and Philipp2011; Lee et al., Reference Lee, Hulman, Musci and Stang2015), and separation of mother and infant at a critical time for bonding (Abrahams et al., Reference Abrahams, MacKay-Dunn, Nevmerjitskaia, MacRae, Payne and Hodgson2010; Wiles et al., Reference Wiles, Isemann, Ward, Vinks and Akinbi2014). Lengthy periods of hospitalization are often required due to the need for pharmacologic management of withdrawal symptoms (Jansson et al., Reference Jansson, Velez and Harrow2009; Hudak and Tan, Reference Hudak and Tan2012). Furthermore, decreased rates of breastfeeding (Wachman et al., Reference Wachman, Byun and Philipp2010; Tsai and Doan, Reference Tsai and Doan2016) and involvement in the child protection system (O’Donnell et al., Reference O’Donnell, Nassar, Leonard, Hagan, Mathews, Patterson and Stanley2009) are additional negative outcomes associated with NAS.
Although not all substance-exposed infants require pharmacologic treatment, a substantial number (60–80%) do require treatment to manage withdrawal (Kocherlakota, Reference Kocherlakota2014). Pharmacologic treatment is diverse and is often contingent on physician practices or specific organizational protocols (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015) as no universal pharmacologic treatment has been established. Methadone or oral morphine are recommended as a first-line pharmacologic treatment for opioid withdrawal, although clonidine and buprenorphine have also been administered to manage the symptoms (McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016).
Recent studies identify that the practice of continuing pharmacologic weaning for NAS out of the hospital setting has been implemented in some healthcare institutions (Saunders et al., Reference Saunders, King, Smith, Buchheit, Cook, Edds and Mefford2014; Kaltenbach and Jones, Reference Kaltenbach and Jones2016; Kraft et al., Reference Kraft, Stover and Davis2016). In particular, in a study evaluating quality improvement for NAS, Patrick et al. (Reference Patrick, Schumacher, Horbar, Buus-Frank, Edwards, Morrow, Ferrelli, Picarillo and Soll2016) found that 34% of infants from 199 centers were discharged from the hospital on medication to be weaned in a home environment. However, there is no consensus or standard of care to guide outpatient weaning (Chau et al., Reference Chau, Nguyen, Miladinovic, Lilly, Ashmeade and Balakrishnan2016; Patrick et al., Reference Patrick, Schumacher, Horbar, Buus-Frank, Edwards, Morrow, Ferrelli, Picarillo and Soll2016). Thus, this systematic review was conducted to assess the effectiveness and safety of outpatient weaning for infants with NAS. Effectiveness was determined by synthesizing the results of studies reporting between group comparisons (inpatient versus outpatient weaning) on NAS outcomes. In addition, safety was assessed by making between group comparisons on variables such as adverse events, child welfare involvement, compliance with follow-up, and hospital readmission rates.
Methods
Search strategy
The electronic databases PubMed, Nursing and Allied Health, CINAHL, Evidence-Based Medicine, Web of Science, Medline, and PsychINFO were searched from 1996 to October, 2017. Subject terms used in the search strategy included ‘neonatal abstinence syndrome’ (Mesh) and one of the following additional terms, outpatient treatment, home treatment, or outpatient weaning. To ensure relevant articles had not been missed, the reference lists of included studies were reviewed for additional articles relevant to the initial search.
Study selection
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). Studies were eligible for inclusion in the review if they fulfilled the following criteria: (1) reported original data on outcomes related to the effectiveness or safety of outpatient weaning for infants with NAS, (2) the infants were discharged from hospital primarily receiving opioid pharmacologic treatment for NAS, (3) the study method included any type of quantitative design that included an inpatient comparison group (infants receiving pharmacologic treatment for NAS in-hospital only), and (4) the articles were published in English in a peer-reviewed journal. For the purpose of this review, NAS was defined as a postnatal withdrawal syndrome in infants that were exposed to opioids in utero (McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016). Thus, NAS in infants exclusively from substances other than opioids (eg, selective serotonin reuptake inhibitors) were excluded from this review. Additional exclusion criteria included: (1) infants being treated for NAS with paregoric, tincture of opium or diazepam as they are not currently recommended for treatment of NAS and (2) infants who were readmitted to hospital with NAS after discharge. Outpatient weaning refers to infants who were initiated on pharmacologic treatment in hospital and received continued pharmacologic treatment for weaning as an outpatient.
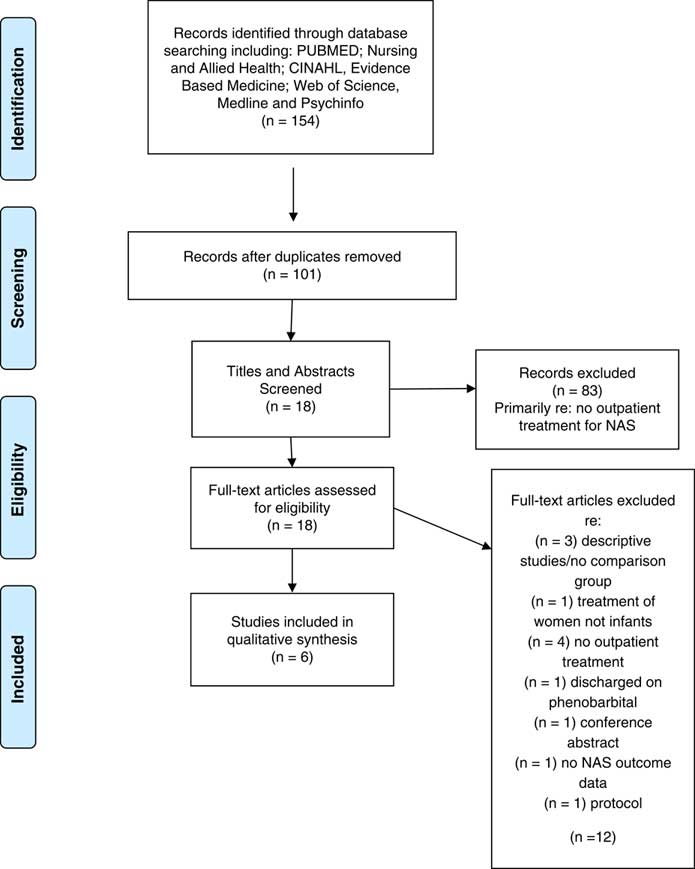
The first author entered all studies from the search into the Zotero Reference Manager. Duplicates were removed and remaining studies were screened for inclusion based on the title, abstract and full text (see Figure 1). Articles that did not meet the inclusion and exclusion criteria were eliminated for further review. The second author screened the excluded articles to ensure accurate exclusion.
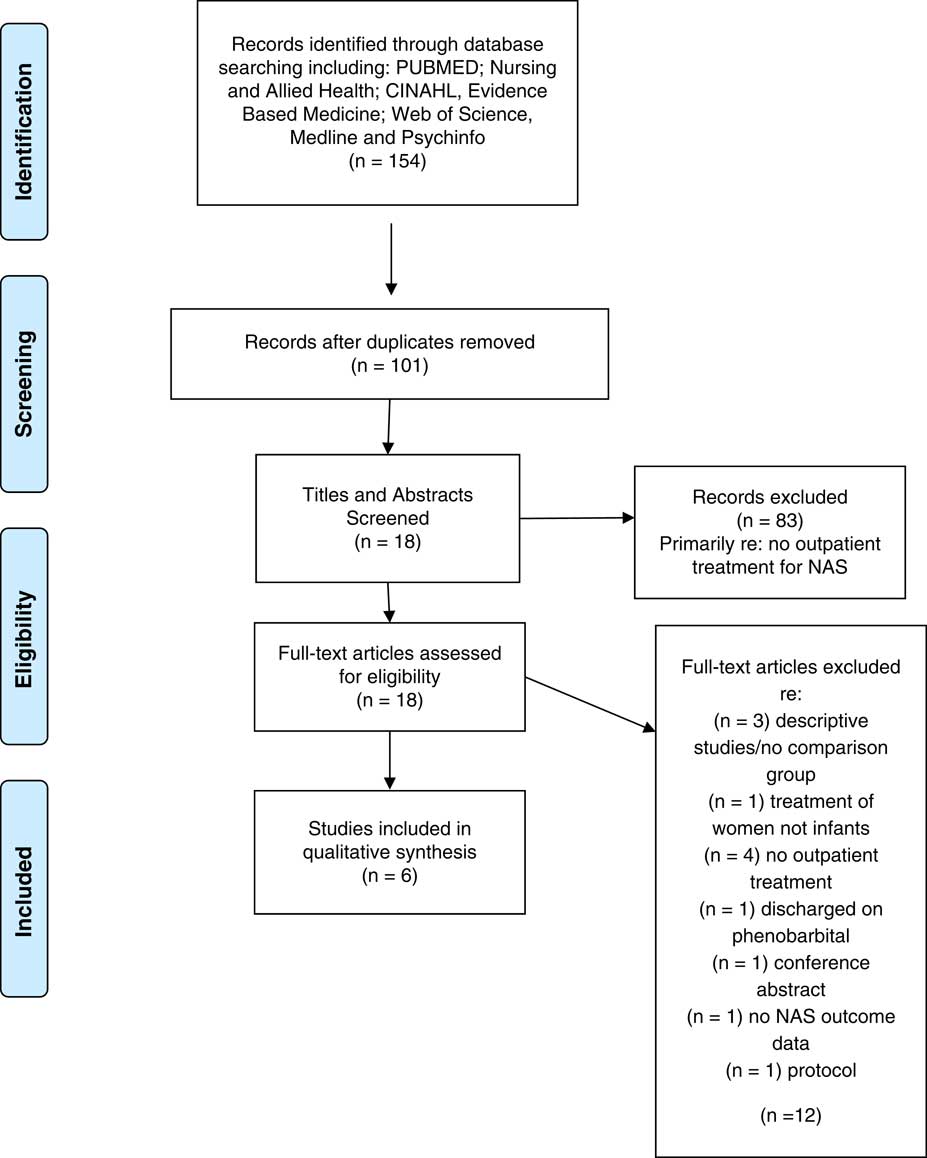
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flow diagram: outpatient weaning
Data extraction
Data from full-text studies was extracted by the principal author and entered into a data extraction template developed for the systematic review in order to capture all relevant details. The template included the authors’ names, date of publication, purpose, study design, number of participants, pharmacologic treatment type, neonatal outcomes, and safety data. The second author also independently extracted data onto a template. The extracted data were then compared and differences were discussed, referring back to the article until an agreement was obtained. A research assistant reviewed the final data extraction template for accuracy or omissions. The data were synthesized narratively as meta-analysis was not possible due to the heterogeneity of the included study samples and outcomes evaluated.
Assessment of methodological quality
Articles selected for inclusion were assessed for methodological quality by two independent reviewers (K.M. and a graduate student) using the Joanna Briggs Institute (JBI) standardized critical appraisal checklist for cohort studies (Joanna Briggs Institute, 2014). Studies were rated as having a low, moderate, or high risk of bias based on participant selection, measurement of exposure (NAS) and outcomes, confounding factors, and follow-up. The independent assessments were compared and any disagreements were resolved through discussion with the primary author (J.M.O.). No studies were eliminated based on the critical appraisal.
Results
The search identified 154 studies, of which 18 provided information related to NAS and outpatient weaning. Further assessment of the inclusion and exclusion criteria eliminated 12 of the studies due to: descriptive studies/no comparison group (n=3); treatment of women not infants (n=1); no outpatient treatment (n=4); discharged on phenobarbital (n=1); no NAS outcome data (n=1); conference abstract (n=1); and published protocol (n=1). A total of six articles met all criteria and are included in the review. See Figure 1 for the flow diagram.
Study characteristics
The characteristics of the six included studies are provided in Table 1. All of the studies were retrospective chart reviews published between 2012 and 2015 and were conducted in the United States (n=3), Canada (n=1), and Australia (n=2). For three of the studies, the primary purpose was to evaluate outpatient weaning for infants with NAS. Whereas the other three studies reported on a subset of infants who received outpatient weaning, although it was not the primary purpose or outcome of the study. Sample sizes ranged from 80 to 981, with a median of 130 participants. Recruitment settings were hospitals that provided care for infants with NAS.
Table 1 Characteristics of included studies
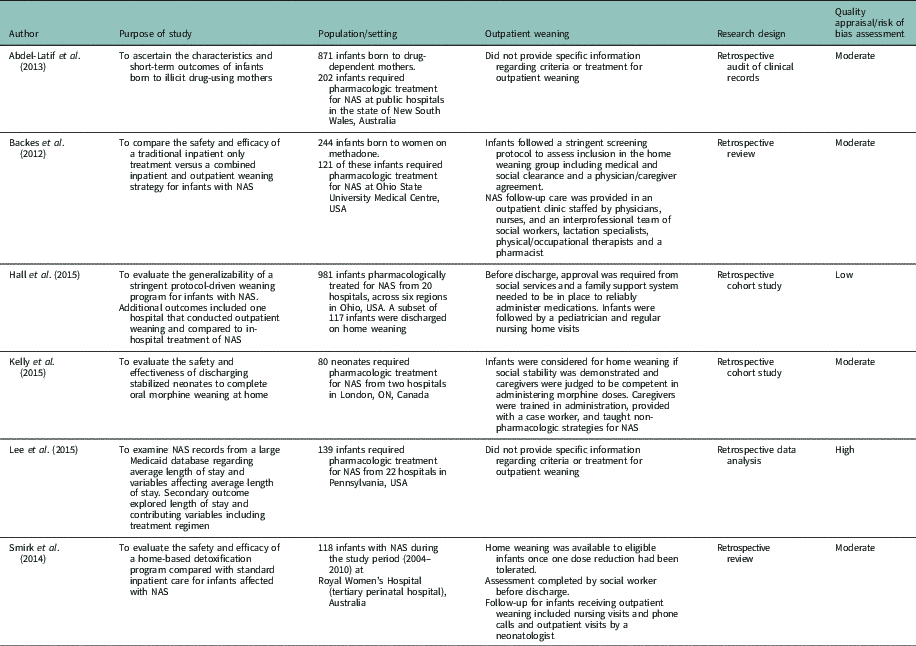
NAS=neonatal abstinence syndrome.
The studies evaluated within this review outlined various practices in the care of infants with NAS. Most studies identified a detailed discharge plan for infants beginning a home weaning program. While all of the studies that indicated the use of a discharge plan required established medical follow-up from a neonatologist or pediatrician, the remaining elements were diverse. The hospital practices included medical stabilization (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015); social stability established by social work (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kelly et al., 2015); a physician/caregiver agreement (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012) confirmed family and social support (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kelly et al., 2015); and nursing home visits or calls post-discharge (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015).
The primary medications used for pharmacologic treatment of NAS were morphine (n=4), methadone (n=1), or either morphine or methadone (n=1) (see Table 2). Phenobarbitone was used as monotherapy for a very small number of infants (eg, <5%), and as a second (eg, adjunctive) medication during home weaning in two studies (Abdel-Latif et al., Reference Abdel-Latif, Oei, Craig and Lui2013; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). The Finnegan or modified version of the Finnegan Scoring tool was used to assess symptoms of NAS and guide treatment in all of the studies. However, it was unclear if they were the same tools as the number of items and/or modifications were not specified. This reflects the diversity of the studies included in the systematic review in terms of treatment (medication, weaning protocols) and outcome measures and participants (eg, mothers in treatment, polysubstance use, term/premature infants). Outcomes measured for the systematic review included: (1) length of hospital stay, (2) duration of treatment, (3) cumulative dose of pharmacologic treatment, (4) healthcare costs, (5) breastfeeding, (6) adverse events, and (7) follow up/child welfare involvement.
Table 2 Synopsis of weaning and outcomes
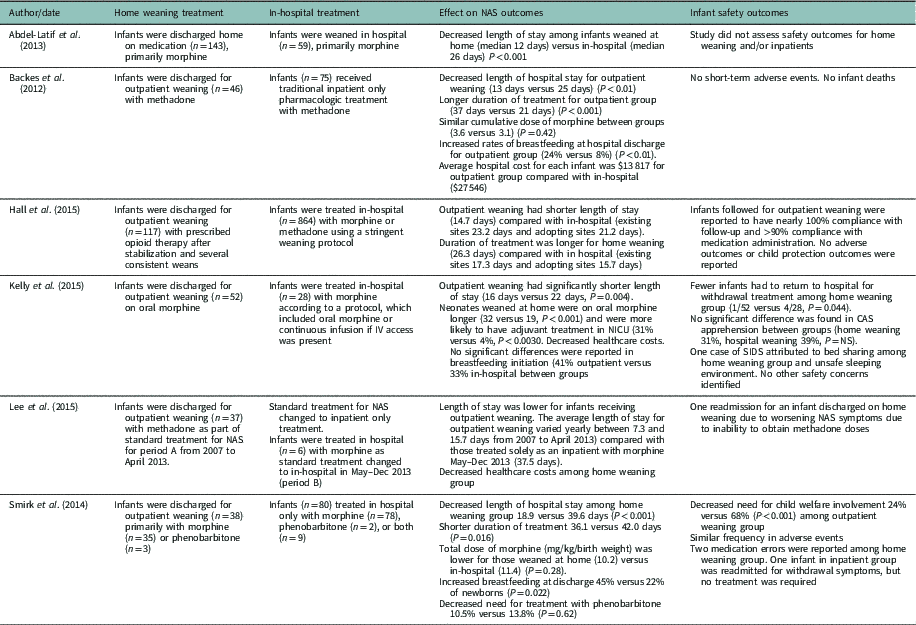
NAS=neonatal abstinence syndrome; NICU=neonatal intensive care unit; CAS=children’s aid society; SIDS=sudden infant death syndrome.
Methodological quality
Overall, the majority (n=4) of the included studies were identified to have a moderate risk of bias (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Abdel-Latif et al., Reference Abdel-Latif, Oei, Craig and Lui2013; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Kelly et al., Reference Kelly, Knoppert, Roukema, Rieder and Koren2015) (see Table 1). One study was identified at low risk of bias (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015) and one with high risk of bias (Lee et al., Reference Lee, Hulman, Musci and Stang2015). Selection bias was present among all studies as the groups were not similar at the outset. Most of the studies identified that infants receiving home weaning had to meet certain discharge criteria for eligibility, which typically included family stability. Likewise, a lack of reporting and/or controlling for confounding variables was present in the majority of studies. Many of the studies reported on baseline characteristics of infants and mothers such as gestation, birth weight, smoking that may affect NAS symptoms; however, other potential confounding factors such as maternal drug and non-pharmacologic treatment for NAS were not controlled for in the analysis. While all studies identified using the Finnegan Scoring Tool or Modified Finnegan to assess NAS and guide treatment, no studies reported on psychometric data regarding the reliability or validity of the tool. Finally, for many of the outcomes it was unclear whether there was a loss to follow-up and how many infants were included in the final analyses.
Home weaning and NAS outcomes
Of the six studies included, all reported on one or more NAS outcomes including length of hospital stay, duration of treatment, healthcare costs and breastfeeding (see Table 2). All studies found that there was a significantly reduced length of hospital stay associated with home weaning. Among infants who received outpatient weaning, the shortest length of stay was 7.3 days (Lee et al., Reference Lee, Hulman, Musci and Stang2015) and longest was 18.9 days (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). For infants who received only in-hospital weaning, the length of stay was much longer, ranging from 15.7 days (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015) to 39.6 days (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). With the decreased length of stay, considerable cost savings were reported among the three studies that evaluated healthcare expenditures (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Kelly et al., 2015; Lee et al., Reference Lee, Hulman, Musci and Stang2015).
While length of stay was reduced, the total duration of opioid treatment for infants who received the outpatient weaning was often longer. In three out of four studies, researchers found that infants who received home weaning received opioid treatment for a longer time period compared with in-hospital treatment (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kelly et al., 2015). Only, Smirk et al. (Reference Smirk, Bowman, Doyle and Kamlin2014) found that the total length of treatment was lower for home weaning infants (36.1 days) compared with in-hospital (42 days, P=0.016). Despite the variations in the duration of treatment among studies, no significant differences were found between groups related to cumulative dosage of morphine (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). Smirk et al. (Reference Smirk, Bowman, Doyle and Kamlin2014) found a lower cumulative dose of morphine (mg/kg/birth weight) for those weaned at home (10.2) compared to in-hospital (11.4), although not statistically significant (P=0.28). Whereas Backes et al. (Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012), who reported longer treatment duration for outpatient weaning (37 versus 21 days, P=0.001), found no significant differences between groups regarding the cumulative morphine doses (mg/kg) (P=0.42).
Three studies reported on rates of breastfeeding. In two of the studies, researchers identified significant differences in breastfeeding rates between groups (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). Smirk et al. (Reference Smirk, Bowman, Doyle and Kamlin2014) found that infants in the outpatient weaning group were almost three times more likely to have been breastfeed upon discharge (n=15, 45%) compared with infants in the hospital-based group (n=18, 22%) (OR 2.9, 95% CI: 1.2–6.8). Similarly, Backes et al. (Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012) found that discharge rates of breastfeeding were significantly higher at hospital discharge for the home weaning group (24% versus 8%, P<0.01). However, Kelly et al. (2015) found breastfeeding initiation rates were similar between groups with 17 (41%) breastfeeding in the outpatient weaning group and seven (33%) breastfeeding in the hospital only group.
Home weaning and safety
Overall, few adverse events were associated with home weaning. No adverse events were reported in two studies (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015), whereas Smirk et al. (Reference Smirk, Bowman, Doyle and Kamlin2014) found a similar frequency of adverse events between groups. Adverse events that were reported among infants on home weaning included one readmission for NAS due to two missed methadone doses (Lee et al., Reference Lee, Hulman, Musci and Stang2015) and one case of sudden infant death syndrome attributed to unsafe sleeping (Kelly et al., 2015). Comparatively, infants weaned in hospital were more often readmitted for withdrawal treatment after discharge than those weaned at home (4/28 versus 1/52, P=0.44) (Kelly et al., 2015). Further, a higher proportion of infants in the hospital group required child protection involvement (OR 0.015, 95% CI: 0.06–0.36) or foster care (OR 0.13, 95% CI: 0.03–0.58) when compared with infants weaned at home.
Positive outcomes were also identified regarding outpatient follow-up and child welfare involvement for infants who received home weaning. Outpatient clinic compliance was high with nearly 100% adherence to follow-up appointments and >90% compliance with medication administration (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015). In addition, there was a decreased need for child welfare involvement for the home weaning group compared with in-hospital (24% versus 68%, P<0.001) (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014).
Discussion
This is the first systematic review to assess the effectiveness and safety of outpatient pharmacologic weaning for infants with NAS. Overall, all studies consistently identified that outpatient weaning for select infants, was associated with shorter hospital stays compared with infants weaned in-hospital only. These findings were consistent regardless of the pharmacologic agent used to wean (methadone, morphine) and the healthcare provider regimen for follow-up. Furthermore, three of the six studies (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Kelly, Reference Kelly, Knoppert, Roukema, Rieder and Koren2015; Lee et al., Reference Lee, Hulman, Musci and Stang2015) reported a reduction in healthcare expenditures for infants weaned at home; however, only one study provided cost estimates of ~$11 000 in healthcare savings for each neonate (Kelly, Reference Kelly, Knoppert, Roukema, Rieder and Koren2015). This review also identified that adverse events were rare and compliance to follow-up treatment was high among those who received outpatient weaning.
Decreased length of hospital stay is an important development given that the length of hospital stay for infants with NAS has remained relatively unchanged in over a decade (Patrick et al., Reference Patrick, Schumacher, Benneyworth, Krans, McAllister and Davis2012; Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015). This is substantial as Patrick et al. (Reference Patrick, Schumacher, Benneyworth, Krans, McAllister and Davis2012) estimated the average costs of NAS as $53 400 per infant (95% CI: $49 000–$57 700), while Tolia reported that 4% of all NICU days across the United States are attributed to NAS. With the incidence of NAS and related healthcare expenditures on the rise (Patrick et al., Reference Patrick, Schumacher, Benneyworth, Krans, McAllister and Davis2012; Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015), a reduction in the period of hospitalization for infants with NAS will invariably lead to a reduction in acute healthcare-related expenditures.
Despite the improvements to the length of hospital stay for infants receiving outpatient weaning, concerns exist regarding the longer duration of outpatient pharmacologic treatment found in three of the studies (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kelly et al., 2015). A longer duration of pharmacologic treatment requires careful consideration given that the long-term outcomes of prolonged opioid treatment are unclear (Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kraft et al., Reference Kraft, Stover and Davis2016). However, while the duration of treatment was longer, the cumulative dose of pharmacologic treatment did not differ between the inpatient and outpatient groups in two of the three studies that measured cumulative dose (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). Thus, the longer duration of treatment did not directly translate into receiving a higher dosage of the medication. The longer duration is likely reflective of a slower taper, which may be advantageous for infants receiving outpatient weaning as few infants returned to hospital for further NAS treatment (Kelly et al., 2015). These findings support the assertion by Hall et al. (Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015) that the use of evidence-based protocols for the management of NAS are needed to improve neonatal outcomes. Thus, further evaluation of weaning protocols and the length of opioid treatment is needed.
Overall, this review found that serious short-term adverse events were rare (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Hall et al., Reference Hall, Wexelblatt, Crowley, Grow, Jasin, Klebanoff, McClead, Meinzen-Derr, Mohan, Stein and Walsh2015; Kelly et al., 2015; Lee et al., Reference Lee, Hulman, Musci and Stang2015) and there was a high rate of compliance with outpatient follow-up. Furthermore, findings revealed that infants receiving outpatient weaning more frequently remained in the care of their biological parents when compared with in-hospital only groups (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). Among the majority of studies, very few child protection concerns were reported for infants weaned at home. However, one study reported a high rate of infant apprehensions in both the in-hospital and outpatient weaning group (Kelly et al., 2015). While all institutions had eligibility criteria for outpatient weaning, the criteria were diverse, and optimal conditions for infant safety have not been established. These are important considerations given that research has found infants with NAS are at a greater risk for involvement with the child welfare system at some point in their early development (O’Donnell et al., Reference O’Donnell, Nassar, Leonard, Hagan, Mathews, Patterson and Stanley2009).
The findings from this review identified higher rates of breastfeeding for infants who were receiving outpatient weaning for NAS (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014). However, the mechanism for improved breastfeeding outcomes found in these studies is unknown. Maintaining the mother–infant dyad may have positively influenced breastfeeding outcomes and/or the engagement with diverse primary care providers involved in the care of infants receiving outpatient weaning. The differences between groups may also be reflective of selection bias with mothers in the in-hospital weaning group more often using multiple substances (Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014), which is considered a contraindication to breastfeeding (Reece-Stremtan and Marinelli, Reference Reece-Stremtan and Marinelli2015). Regardless, these are noteworthy findings as breastfeeding has been associated with positive impacts on NAS outcomes including delayed symptom onset (Liu et al., Reference Liu, Juarez, Nair and Nanan2015), reduced incidence, decreased severity of symptoms, and decreased pharmacotherapy compared with infants who are not breastfed (Pritham, Reference Pritham2013; Welle-Strand et al., Reference Welle-Strand, Skurtveit, Jansson, Bakstad, Bjarkø and Ravndal2013). As such, breastfeeding should be recommended as a supportive non-pharmacologic treatment for NAS among stabilized mothers (Bagley et al., Reference Bagley, Wachman, Holland and Brogly2014; Kaltenbach and Jones, Reference Kaltenbach and Jones2016; McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016).
Despite the potential benefits associated with breastfeeding, rates remained low in this population (Backes et al., Reference Backes, Backes, Gardner, Nankervis, Giannone and Cordero2012; Smirk et al., Reference Smirk, Bowman, Doyle and Kamlin2014; Kelly et al., 2015). This finding is consistent with previous research reporting on low rates of breastfeeding for infants with NAS (Wachman et al., Reference Wachman, Byun and Philipp2010). Many infants requiring pharmacologic treatment for NAS are treated in a special care nursery in isolation from their mothers (Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015; Uebel et al., Reference Uebel, Wright, Burns, Hilder, Bajuk, Breen, Abdel-Latif, Feller, Falconer, Clews, Eastwood and Oei2015; McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016), thus inhibiting exclusive breastfeeding (Flacking et al., Reference Flacking, Lehtonen, Thomson, Axelin, Ahlqvist, Moran, Ewald and Dykes2012). Additional barriers to breastfeeding for infants with NAS may include NAS symptoms (eg, irritability, tachypnea, increased tone) and lack of information or discouragement of breastfeeding by healthcare professionals (McQueen and Murphy-Oikonen, Reference McQueen and Murphy-Oikonen2016; Tsai and Doan, Reference Tsai and Doan2016). Infants treated in a home environment have less structural barriers to impede breastfeeding which may positively influence breastfeeding rates among infants with NAS.
Additional benefits may be associated with outpatient weaning that have not been evaluated in this systematic review. Lengthy hospitalization for infants with NAS is associated with the separation of mother and infant at a critical time for infant development and bonding (Cleary et al., Reference Cleary, Donnelly, Strawbridge, Gallagher, Fahey, White and Murphy2011; Tolia et al., Reference Tolia, Patrick, Bennett, Murthy, Sousa, Smith, Clark and Spitzer2015; Uebel et al., Reference Uebel, Wright, Burns, Hilder, Bajuk, Breen, Abdel-Latif, Feller, Falconer, Clews, Eastwood and Oei2015). Given that the postnatal period is a crucial time for maternal-infant bonding and subsequent attachment (Crouch and Manderson, Reference Crouch and Manderson1995; Shannon et al., Reference Shannon, Blythe and Peters2016), a treatment model that decreases the separation of mother and infant may positively influence the maternal-child relationship. Thus, implementing outpatient weaning for NAS has the potential to empower mothers to assume the caregiver role in a natural environment and develop a bond with their newborn, while facilitating recovery from NAS symptoms.
Limitations
All of the included studies were retrospective in nature and relied on the accuracy of medical records. Many studies had small sample sizes of infants that received home weaning and for some studies, evaluating home weaning was not the primary purpose. Furthermore, while the included studies discuss reduced healthcare expenditures related to decreased length of hospital stay, there was a lack of clarity of how costs were measured in two of the three studies that evaluated this outcome. In addition, the critical appraisal identified that the majority of included studies were at moderate of bias. Thus, the generalizability of the findings is limited to a select group of mothers and infants who may be appropriate for home weaning. As a result of the identified limitations, results of the systematic review need to be interpreted cautiously.
Implications for practice
The benefits of outpatient weaning for length of hospital stay and related healthcare expenditures have influenced some healthcare institutions and provider groups to explore outpatient weaning as a treatment option for select infants with NAS. The presence of few adverse events and safety concerns likely reflects the well-developed protocols used to guide primary healthcare providers’ decision making in many of the included studies. Given the social risk factors associated with substance use (Meyer et al., Reference Meyer, McWey, McKendrick and Henderson2010; Traube, Reference Traube2012), hospitals implementing outpatient weaning need to consider social stability and follow-up services available to infants with NAS. Social stability may require a psychosocial assessment from an in-hospital social worker before consideration for discharge. Follow-up services that are inclusive of pharmacologic management and/or monitoring, nursing, social, or familial supports may also be beneficial to both mother and infant. Given the diversity of approaches to establish safety criteria before discharge of infants to continue weaning in a home environment, there is a need for further research to establish optimal eligibility criteria to promote infant safety.
Further, primary care providers supporting families receiving home weaning need protocols in place to guide decision making regarding referral to other disciplines (eg, physicians, social work, lactation specialists, nurses, etc.). Given that infants and families undergoing home weaning for NAS require both pharmacologic treatment and social follow up, there is a role for primary healthcare providers to assume the care of infants receiving outpatient weaning for NAS. However, this role requires further development to ensure infant safety and well-being.
Implications for future research
Findings from this systematic review have several research implications. Larger, prospective studies are required to rigorously assess the effectiveness and safety of home weaning. Further research is also required to identify whether there is an optimal protocol to guide treatment with an emphasis on evaluating duration of treatment, cumulative dose and safety (eg, adverse events). Moreover, consideration should be given to the challenges of identifying which NAS outcomes are important to measure. Improving length of stay is important from a cost perspective; however, future research is needed to conduct robust economic evaluations to ascertain healthcare savings for infants weaned at home. Furthermore, duration of treatment and cumulative dose have potential negative impacts on infants and must be considered. Further exploration of eligibility criteria for home weaning that optimizes infant safety is also necessary before recommending this treatment approach. In addition, given that home weaning is a relatively new practice, qualitative research that explores the experiences of mothers is needed to understand mother’s perception of their role in outpatient weaning, effectiveness of supports, and efficacy in providing this treatment in a home environment. Finally, further research is required on the effect of the involvement of a multi-disciplinary team on outpatient weaning.
Conclusion
The findings from this systematic review suggest that outpatient weaning for select infants with NAS was effective in reducing the length of hospital stay with minimal adverse outcomes or need for child welfare involvement. However, the duration of pharmacologic treatment was typically longer for infants weaned at home and warrants further evaluation due to the unknown long-term effects of opioids on infants. Furthermore, given the reduction in the length of hospital stay, outpatient weaning may be effective in reducing related healthcare costs, however, large-scale trials are required to establish cost-effectiveness.
Acknowledgments
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.