Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose or carbohydrate intolerance with onset or first recognition during pregnancy (ACOG Committee on Practice Bulletins-Obstetrics, 2004; International Association of Diabetes and Pregnancy Study Groups Consensus Panel et al., Reference Metzger, Gabbe, Persson, Buchanan, Catalano, Damm, Dyer, Leiva, Hod, Kitzmiler, Lowe, McIntyre, Oats, Omori and Schmidt2010). GDM is among the most common medical disorders of the perinatal period affecting up to 21% of women globally (Bener et al., Reference Bener, Saleh and Al-Hamaq2011). A current study estimated that the international prevalence of hyperglycemia in pregnancy in women (20–49 years) is 16.9%, or 21.4 million live births in 2013 (Guariguata et al., Reference Guariguata, Linnenkamp, Beagley, Whiting and Cho2014). With an ever-increasing prevalence worldwide, GDM constitutes a major public health concern considering its adverse effects on maternal and neonatal outcomes (Crowther et al., Reference Crowther, Hiller, Moss, McPhee, Jeffries and Robinson2005; Metzger, Reference Metzger2007).
GDM is a well-known risk factor for developing type 2 diabetes in the future. Women with a history of GDM have a sevenfold increased risk of developing type 2 diabetes within 10 years of the affected pregnancy (Bellamy et al., Reference Bellamy, Casas, Hingorani and Williams2009). These women also have an increased risk for cardiovascular diseases. Moreover, GDM is a well-established risk factor for a wide range of complications during pregnancy and birth, to include pre-eclampsia, hypertension, cesarean section, and preterm birth (Metzger, Reference Metzger2007). Importantly, infants of mothers with GDM have several short- and long-term adverse outcomes including macrosomia, preterm birth, metabolic disorders such as hypoglycemia, hypocalcemia, hyperbilirubinemia, respiratory disorders, obesity, and cognitive impairments (Crowther et al., Reference Crowther, Hiller, Moss, McPhee, Jeffries and Robinson2005; Metzger, Reference Metzger2007; Mitanchez et al., Reference Mitanchez, Yzydorczyk and Simeoni2015).
Another common condition of the perinatal period is depression. Between 14 and 23% of pregnant women will experience a depressive disorder during pregnancy (Gaynes et al., Reference Field2005) and 15–20% of women will develop postpartum depression (PPD) after giving birth (Davé et al., Reference Davé, Petersen, Sherr and Nazareth2010). A latest review reported its prevalence to be 1.9–82.1% and 5.2–74.0% in developing and developed countries, respectively, using a self-reported questionnaire. Its prevalence has also been reported to vary from 0.1 to 26.3% using a structured clinical interview (Norhayati et al., Reference Norhayati, Hazlina, Asrenee and Emilin2015). Perinatal depression has detrimental consequences for the lives of women, children, and their families. Prenatal depressive symptomatology has been linked to poor maternal self-care and outcomes. Pregnant women who experience depressive symptoms are less likely to look for proper medical care during pregnancy (Leung and Kaplan, Reference Leung and Kaplan2009). Untreated PPD can have long-lasting negative effects on the woman, the maternal role, the mother–child relationship, child development, and the marital relationship (Murray et al., Reference Murray, Fiori-Cowley, Hooper and Cooper1996; Weinberg and Tronick, Reference Weinberg and Tronick1998; Field, Reference El-Den, O’Reilly and Chen2010).
There is evidence of a bidirectional relationship between depression and diabetes. Depression earlier in life increases the risk for development of type 2 diabetes (Golden et al., Reference Gaynes, Gavin, Meltzer-Brody, Lohr, Swinson, Gartlehner, Brody and Miller2008) and diabetes-specific complications are associated with a higher risk of subsequent depression (Katon et al., Reference Katon, Russo, Lin, Heckbert, Ciechanowski, Ludman and Von Korff2009). Diabetes doubles the likelihood of comorbid depression, which is present in ~30% of patients with type 1 or type 2 diabetes (de Groot et al., Reference Golden, Lazo, Carnethon, Bertoni, Schreiner, Diez Roux, Lee and Lyketsos2001).
Less is known about the relationship between GDM and perinatal depressive symptoms. Previous studies have identified an association between GDM and prenatal depressive symptoms (Kozhimannil et al., Reference Kozhimannil, Pereira and Harlow2009); however, contradicting findings have also been reported (Katon et al., Reference Katon, Russo, Gavin, Melville and Katon2011; Byrn and Penckofer, Reference Byrn and Penckofer2015). Moreover, a number of studies indicate an association between GDM and PPD (Dalfrà et al., Reference Dalfrà, Lapolla, Masin, Giglia, Dalla Barba, Toniato and Fedele2001), while others have not confirmed this association (Kim et al., Reference Kim, Brawarsky, Jackson, Fuentes-Afflick and Haas2005; Besser et al., Reference Besser, Priel, Flett and Wiznitzer2007; Halkoaho et al., Reference Halkoaho, Pietilä, Huopio, Sintonen and Heinonen2010; Al-Shahrani et al., Reference Al-Shahrani, Al-Sunaidi, Al-Amri, Al-Maswary and Al-Gelban2011; Liu and Tronick, Reference Liu and Tronick2013).
Purpose
The main aim of this study is to examine the association between GDM with prenatal depressive symptoms in the third trimester of pregnancy and PPD symptoms in the first week postpartum, in a sample of pregnant women in Greece. To the authors’ knowledge, no similar research has been conducted in Greece.
Methods
Sample
Participants were recruited from the practice of collaborating obstetricians in Athens, Greece. The study sample consisted of a total of 117 women on their third trimester of pregnancy who received regular prenatal care. Eligible participants were women in their third trimester of pregnancy of Greek origin or fluent in Greek language, who were provided with a detailed description of the study procedures and signed a written informed consent before the inclusion in the study. Exclusion criteria were active psychotic symptoms, organic brain pathology, and intellectual disability. The study protocol was approved by the assembly of Athens University Medical School (approval number: 6761) for scientific and ethical standards.
Measures
Data were collected at two time points: (1) during the third trimester (anytime between 32nd and 35th week) of pregnancy; and (2) within the first week postpartum. Demographic and general health information were assessed during the prenatal assessment, while the Edinburgh Postnatal Depression Scale (EPDS) (Cox et al., Reference Cox, Holden and Sagovsky1987) and obstetric information at both time points. Participants were asked to complete a package of self-report measures including socio-demographic information, a detailed obstetric history, and current health status.
Prenatal depressive and PPD symptoms were assessed with the EPDS. The EPDS is a self-report questionnaire assessing the severity of depression symptoms during pregnancy and postpartum. It consists of 10 items, each rated on a four-point scale; items three and five through 10 are reverse scored. Range of possible score is between 0 and 30, and higher scores suggest greater severity of depressive symptoms. EPDS is a widely used screening tool for perinatal depression with satisfactory psychometric properties both in prenatal and postnatal populations (Cox et al., Reference Cox, Holden and Sagovsky1987). The Greek version of EPDS has been validated for perinatal use (Leonardoua et al., Reference Leonardoua, Zervas, Papageorgiou, Marks, Tsartsara, Antsaklis, Christodoulou and Soldatos2009). For the purposes of this study, diagnosis of probable major depression was set at the well-validated cut-off point of 12/13 (Cox et al., Reference Cox, Holden and Sagovsky1987).
Data analysis
Descriptive statistics were calculated for initial data analysis and demographic, clinical, and obstetric data were compared using Student’s t, Mann–Whitney U, χ 2, or Fisher’s exact tests, as appropriate. Differences in mean EPDS scores between GDM and non-GDM groups were explored both during pregnancy and postpartum. Because EPDS scores were non-normally distributed as evidenced by plotted data and significant Kolmogorov–Smirnov normality test (P<0.001), non-parametric tests were used (Mann–Whitney U). Logistic regression analyses were also conducted, with EPDS score as a categorical dependent variable (probable major depressive disorder (MDD) diagnosis ⩾13 versus no MDD diagnosis ⩽12). We first tested models including pregnancy or postpartum probable MDD diagnosis only in the model, and then models controlling for confounding variables (age, nulliparity, mode of delivery, and neonatal intensive care unit (NICU) admission). Statistical significance level was set at P-value of 0.05. Data management and analysis were performed using SPSS software package version 21.0.
Findings
Table 1 presents the demographic and clinical characteristics of the sample. In brief, the average age of women was 32.6 (SD=4.06) years. The majority was married (95.7%) and predominantly Greek (93.2%), and had completed higher education (65.0%). In addition, 83.8% of them were employed, with most of them (56.4%) having a monthly family income between €1000 and 3000.
Table 1 Descriptive statistics of the original (n=117) and follow-up (n=93) sample
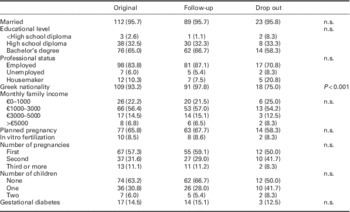
The majority was primiparous (57.3%) with a mean gestational age of 32 weeks. Pregnancy was reportedly planned for 77 (65.8%) of women of the sample, while 10 women (8.5%) had become pregnant through in vitro fertilization. The prevalence of GDM was 14.5% (Table 1). From the initial sample of 117 women who participated in the prenatal data collection time point, 93 (79%) were successfully reassessed in the first week postpartum follow-up. No significant demographic differences were found between these two groups (initial versus follow-up sample) (Table 1).
The median EPDS score during the third trimester of pregnancy was 5.00 (interquartile range (IQR)=6.00) compared with 6.0 (IQR=7.0) in the first week postpartum. Using the validated cut-off of 12/13 for major depression, 12% had a likely diagnosis of depression during pregnancy and 15.1% in the first week postpartum (Table 2).
Table 2 Descriptive statistics of prenatal and postnatal depressive symptoms

IQR=interquartile range; EPDS=Edinburg Postnatal Depression Scale.
A significant association was found between presence of GDM and postpartum EPDS scores (Mann–Whitney U=762.0, Z=2.25, P=0.024). Women with GDM scored higher on the EPDS within the first week postpartum (median=8.00, IQR=8.00) compared with women without GDM (median=5.00, IQR=7.00). No statistically significant relationship between GDM and prenatal EPDS scores was found (Mann–Whitney U=974.5, Z=0.97, P=0.33) (Table 3).
Table 3 Prenatal and postnatal depressive symptoms with health problems during pregnancy

GDM=gestational diabetes mellitus; IQR=interquartile range; EPDS=Edinburg Postnatal Depression Scale.
Probable depression diagnosis=EPDS score ⩾13.
Similar results were reported with depression as a categorical variable (probable diagnosis=EPDS score ⩾13). Significantly, more women with GDM screened positive for depression compared with non-GDM women (35.7 versus 11.4%; χ 2=5.501, P=0.02). Moreover, women with GDM were significantly more likely to screen positive for PPD, even after controlling for confounding variables including age, monthly income, nulliparity, mode of delivery, and NICU admission. GDM was significantly associated with increased odds for PPD (adjusted odds ratio 4.69, 95% CI 1.07–20.64).
Discussion
The aim of the present prospective study was to elucidate on the relationship between GDM and depressive symptoms during pregnancy and postpartum. Our findings revealed a significant association between GDM and depression symptoms in the postpartum period. Interestingly, no relationship was observed between GDM and depression scores in the third trimester of pregnancy.
Our results corroborate previous findings suggesting a link between GDM and PPD, but not antenatal depression. For instance, Kozhimannil et al. (Reference Kozhimannil, Pereira and Harlow2009) found that pre-pregnancy diabetes or GDM was independently associated with new onset of PPD. Similarly, Dalfrà et al. (Reference Dalfrà, Lapolla, Masin, Giglia, Dalla Barba, Toniato and Fedele2001) found that women with type 1 diabetes mellitus and GDM had significantly higher levels of depressive symptoms at eight weeks postpartum compared with controls. More recently, Byrn and Penckofer (Reference Byrn and Penckofer2015) found that women with GDM had higher rates of depression; however, their results were not statistically significant. These results were echoed by Katon et al. (Reference Katon, Russo, Gavin, Melville and Katon2011) who found that neither pre-existing diabetes nor GDM was associated with increased risk of antenatal depression when adjusting for confounding factors.
Several studies, however, have failed to report a relationship between GDM and PPD. For instance, a large, prospective cohort study reported comparable rates of PPD symptoms among women with and without GDM (Kim et al., Reference Kim, Brawarsky, Jackson, Fuentes-Afflick and Haas2005). Another study conducted in Saudi Arabia by Al-Shahrani et al. (Reference Al-Shahrani, Al-Sunaidi, Al-Amri, Al-Maswary and Al-Gelban2011) using the same instrument and time intervals of assessments with our study, found no significant differences in PPD symptoms between women with and without GDM. Moreover, a recent study by Liu and Tronick (Reference Liu and Tronick2013) showed that there was no association between PPD and higher rates of GDM after controlling for socio-demographic variables. A recent prospective study also failed to report a significant association between diabetes (including GDM and or pre-pregnancy diabetes) and PPD; however, women with pre-pregnancy diabetes were more likely to develop PPD compared with those without pre-pregnancy diabetes (Miller et al., Reference Miller, Peri and Gossett2015).
There are several possible explanations for the discrepant results among different studies. First, there are significant variations in the methodology and design, some studies are cross-sectional while others longitudinal, and there is considerable variation in postpartum time point assessment. Moreover, there are no unified diagnostic criteria for GDM and PPD, and thus it is reasonable to expect that the different ways of assessing the symptoms as well as the different time points of assessment can yield mixed findings. Furthermore, variability in sample size and heterogeneity in clinical as well as cultural characteristics of postpartum women may also play a role.
These discrepancies notwithstanding, there are possible mechanisms to support an association between PPD and GDM. Diabetes affects glycemic control and thyroid function, both of which impact the hypothalamic–pituitary–adrenal axis and cortisol levels (Lustman et al., Reference Lustman, Anderson, Freedland, de Groot, Carney and Clouse2000). These hormonal changes may be important contributing factors in the development of depression during the perinatal period (Chen et al., Reference Chen, Lan, Yang and Juang2006; Kammerer et al., Reference Kammerer, Taylor and Glover2006). In addition, the stress of managing an illness that poses risks to the woman and the infant may exacerbate depressive symptoms in pregnant women and mothers (Séguin et al., Reference Séguin, Potvin, St-Denis and Loiselle1995).
Limitations
Our study findings should be interpreted with caution due to some limitations. First, in our study we followed up women in the postpartum only once, a week after they gave birth. Including at least one additional assessment time point perhaps six months following birth would significantly improve the validity of EPDS scores. Second, the sample was relatively small to control for confounders, and during the postpartum reassessment 20.51% of the sample had dropped out. Despite these limitations, additional analysis found no significant differences between the initial and follow-up sample, suggesting that sample attrition followed a random pattern and was not due to systematic biases. In addition, we did not collect anthropometric data, such as pre-pregnancy body mass index; this is an important omission, given that obesity is a well-documented risk factor for GDM (Chu et al., Reference Chu, Callaghan, Kim, Schmid, Lau, England and Dietz2007; Torloni et al., Reference Torloni, Betrán, Horta, Nakamura, Atallah, Moron and Valente2009) and a correlate of depression (Luppino et al., Reference Luppino, de Wit, Bouvy, Stijnen, Cuijpers, Penninx and Zitman2010) and perinatal depressive symptomatology (Molyneaux et al., Reference Molyneaux, Poston, Ashurst-Williams and Howard2014). Furthermore, our sample was recruited from a large urban center and the majority of the participating women had relatively high educational attainment, which may limit the generalizability of our findings to women with different demographic profiles.
Future recommendations
In view of these limitations, we hope that these preliminary results will stimulate further research to establish whether a link exists between PPD symptomatology and GDM. Further research should aim at assessing depression symptoms using structured diagnostic tools in addition to self-report measures, while GDM diagnosis should be based on biological screening rather than self-reports. Large representative samples will permit investigation of the relationship between GDM and perinatal depression adjusting for confounding factors. Assessment should be conducted at multiple time points, during first, second, and third trimester as well as at several time points postpartum (eg, one week, six month, one year).
Conclusion
Our study findings, albeit preliminary, may have important clinical implications. Women with GDM appear to be at risk for developing a mood disorder following childbirth. This finding points toward the need for healthcare providers to closely monitor women with GDM for PPD. There is compelling evidence for the deleterious effects of depression on the mother, the infant, and the entire family system (Murray et al., Reference Murray, Fiori-Cowley, Hooper and Cooper1996; Weinberg and Tronick, Reference Weinberg and Tronick1998; Field, Reference El-Den, O’Reilly and Chen2010). Thus, timely detection of PPD and identification of vulnerable groups to PPD are of great public health significance. Several screening measures for PPD have been developed, with sound psychometric properties and good acceptability and feasibility (El-Den et al., Reference de Groot, Anderson, Freedland, Clouse and Lustman2015).
In conclusion, the evidence from our study suggests that GDM is associated with depressive symptoms in the first week postpartum. Given the preliminary nature of the present study, our results should be replicated in larger, more robust studies.
Acknowledgments
The authors thank all the women who participated in this study.
Authors’ Contribution
P.V. initiated the research, participated in study design and data analysis, conceived the study, designed and carried out the study, participated in data collection, and drafted the manuscript. A.C.S. participated in the study design, manuscript development, and data analysis and interpretation. Z.K. supervised statistical analysis and interpreted the data. E.V. participated in statistical analysis and manuscript preparation. M.M. participated in drafting of the manuscript and critical revision of the manuscript. I.M.Z. supervised the design of the study, data collection, and data analysis and interpretation. All authors read and approved the final manuscript.
Financial Support
None.
Conflicts of Interest
None.






