No CrossRef data available.
Article contents
X-ray powder diffraction data for three new compounds obtained as a result of CO2 capture
Published online by Cambridge University Press: 23 November 2023
Abstract
The field of research related to CO2 capture is significant and really attractive for sustainable green chemistry. Focusing attention on this topic in our research led to obtaining new compounds based on diamines. As a result of the syntheses carried out using aqueous solutions of diamines exposed to the slow action of carbon dioxide from the air, three new monocarbamates were obtained. X-ray powder diffraction data for the obtained compounds: 12-propCO2 (C4H10N2O2) [a = 9.3033(7), b = 9.2485(7), c = 7.4735(7) Å, β = 111.214(7)°, V = 599.46 Å3, Z = 4, space group Ia]; 13-propCO2 (C4H10N2O2) [a = 5.0065(10), b = 12.2093(23), c = 4.9006(10) Å, β = 96.457(18)°, V = 297.65 Å3, Z = 2, space group P21]; and 13-dytekCO2 (C6H14N2O2) [a = 28.374(3), c = 5.1726(9) Å, V = 3606.53 Å3, Z = 18, space group 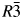 $R\bar{3}$] are reported in this paper.
$R\bar{3}$] are reported in this paper.
- Type
- New Diffraction Data
- Information
- Copyright
- Copyright © The Author(s), 2023. Published by Cambridge University Press on behalf of International Centre for Diffraction Data



