Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Antao, Sytle M.
2013.
Three cubic phases intergrown in a birefringent andradite-grossular garnet and their implications.
Physics and Chemistry of Minerals,
Vol. 40,
Issue. 9,
p.
705.
Antao, Sytle M.
2014.
Schorlomite and morimotoite: what's in a name?.
Powder Diffraction,
Vol. 29,
Issue. 4,
p.
346.
Antao, Sytle M.
and
Round, Stephanie A.
2014.
Crystal chemistry of birefringent spessartine.
Powder Diffraction,
Vol. 29,
Issue. 3,
p.
233.
Antao, Sytle M.
and
Klincker, Allison M.
2014.
Crystal structure of a birefringent andradite–grossular from Crowsnest Pass, Alberta, Canada.
Powder Diffraction,
Vol. 29,
Issue. 1,
p.
20.
Antao, Sytle M.
2014.
Crystal structure of morimotoite from Ice River, Canada.
Powder Diffraction,
Vol. 29,
Issue. 4,
p.
325.
Antao, Sytle M.
2015.
Crystal chemistry of birefringent hydrogrossular.
Physics and Chemistry of Minerals,
Vol. 42,
Issue. 6,
p.
455.
Antao, Sytle M.
Zaman, Mashrur
Gontijo, Vitor L.
Camargo, Eric S.
and
Marr, Robert A.
2015.
Optical anisotropy, zoning, and coexistence of two cubic phases in andradites from Quebec and New York.
Contributions to Mineralogy and Petrology,
Vol. 169,
Issue. 2,
Badar, Manzoor Ahmad
Hussain, Safdar
Niaz, Shanawer
and
Rehman, Saif ur
2016.
Anomalous optical variations in the grossular garnet from the Eden Mills, Belvidere Mountain (Vermont, USA).
Arabian Journal of Geosciences,
Vol. 9,
Issue. 9,
Antao, Sytle M.
and
Dhaliwal, Inayat
2017.
Growth Oscillatory Zoning in Erythrite, Ideally Co3(AsO4)2·8H2O: Structural Variations in Vivianite-Group Minerals.
Minerals,
Vol. 7,
Issue. 8,
p.
136.
Huq, Ashfia
Kirkham, Melanie
Peterson, Peter F.
Hodges, Jason P.
Whitfield, Pamela S.
Page, Katharine
Hűgle, Thomas
Iverson, Erik B.
Parizzi, Andre
and
Rennich, George
2019.
POWGEN: rebuild of a third-generation powder diffractometer at the Spallation Neutron Source.
Journal of Applied Crystallography,
Vol. 52,
Issue. 5,
p.
1189.
Zaman, M.
and
Antao, Sytle
2020.
Crystal Chemistry and Structural Variations for Zircon Samples from Various Localities.
Minerals,
Vol. 10,
Issue. 11,
p.
947.
Zaman, M. Mashrur
and
Antao, Sytle M.
2020.
Crystal Structure Refinements of Four Monazite Samples from Different Localities.
Minerals,
Vol. 10,
Issue. 11,
p.
1028.
Tančić, Pavle
Kremenović, Aleksandar
and
Vulić, Predrag
2020.
Structural dissymmetrization of optically anisotropic Grs64±1Adr36±1Sps2grandite from Meka Presedla (Kopaonik Mt., Serbia).
Powder Diffraction,
Vol. 35,
Issue. 1,
p.
7.
Zaman, M. Mashrur
and
Antao, Sytle M.
2020.
A Possible Radiation-Induced Transition from Monazite-(Ce) to Xenotime-(Y).
Minerals,
Vol. 11,
Issue. 1,
p.
16.
Antao, Sytle M.
2021.
Crystal Chemistry of Six Grossular Garnet Samples from Different Well-Known Localities.
Minerals,
Vol. 11,
Issue. 7,
p.
767.
Umbsaar, Darren A.
and
Antao, Sytle M.
2021.
Structural Variations across Wolframite Solid Solutions, (Fe,Mn)WO4.
Minerals,
Vol. 12,
Issue. 1,
p.
42.
Antao, Sytle M.
2021.
Crystal Structure of an Anisotropic Pyrope Garnet That Contains Two Cubic Phases.
Minerals,
Vol. 11,
Issue. 12,
p.
1320.
Antao, Sytle
2021.
Linear Structural Trends and Multi-Phase Intergrowths in Helvine-Group Minerals, (Zn,Fe,Mn)8[Be6Si6O24]S2.
Minerals,
Vol. 11,
Issue. 3,
p.
325.
Hazrah, Kaveer
and
Antao, Sytle
2022.
Apatite, Ca10(PO4)6(OH,F,Cl)2: Structural Variations, Natural Solid Solutions, Intergrowths, and Zoning.
Minerals,
Vol. 12,
Issue. 5,
p.
527.
Bindhu, Amrithakrishnan
Naseemabeevi, Jawahar I.
and
Ganesanpotti, Subodh
2022.
Distortion and energy transfer assisted tunability in garnet phosphors.
Critical Reviews in Solid State and Materials Sciences,
Vol. 47,
Issue. 5,
p.
621.
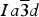 $Ia\overline 3 d$ , and monochromatic synchrotron high-resolution powder X-ray diffraction (HRPXRD) data, which shows a mixture of three distinct cubic phases that are intergrown together and cause birefringence because of strain arising from small structural mismatch. This mixture of three cubic phases was not observed by any other experimental technique. These results have many implications, including garnet phase transitions from cubic to lower symmetry in the mantle, which has important geophysical consequences.
$Ia\overline 3 d$ , and monochromatic synchrotron high-resolution powder X-ray diffraction (HRPXRD) data, which shows a mixture of three distinct cubic phases that are intergrown together and cause birefringence because of strain arising from small structural mismatch. This mixture of three cubic phases was not observed by any other experimental technique. These results have many implications, including garnet phase transitions from cubic to lower symmetry in the mantle, which has important geophysical consequences.