Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Barrio, María
López, David O.
Tamarit, Josep Ll.
Negrier, Philippe
and
Haget, Yvette
1995.
Degree of miscibility between non-isomorphous plastic phases: binary system NPG (neopentyl glycol)-TRIS[tris(hydroxymethyl)aminomethane].
J. Mater. Chem.,
Vol. 5,
Issue. 3,
p.
431.
Barrio, M.
López, D.O.
Tamarit, J.Ll.
and
Haget, Y.
1995.
Plastic molecular alloys: binary system 2-methyl-2-nitro-1-propanol/2,2-dimethyl-1,3-propanediol.
Materials Research Bulletin,
Vol. 30,
Issue. 6,
p.
659.
Hashimoto, Masao
Tajima, Fukue
and
Yamamura, Kimiaki
2002.
Tetrameric O–H⋯O hydrogen-bond systems accompanied by C–H⋯O hydrogen-bonds in the crystal of 1,4-bis(hydroxymethyl)triptycene: an X-ray study.
Journal of Molecular Structure,
Vol. 616,
Issue. 1-3,
p.
129.
Chandra, D.
Mandalia, H.
Chien, W.-M.
Lindle, D. W.
and
Rudman, R.
2002.
Solid-Solid Phase Transition in Trimethylolpropane (TRMP).
Zeitschrift für Physikalische Chemie,
Vol. 216,
Issue. 12,
Chandra, D.
Chien, W.-M.
Gandikotta, V.
and
Lindle, D. W.
2002.
Heat Capacities of “Plastic Crystal” Solid State Thermal Energy Storage Materials.
Zeitschrift für Physikalische Chemie,
Vol. 216,
Issue. 12,
Chellappa, Raja
and
Chandra, Dhanesh
2003.
Phase diagram calculations of organic “plastic crystal” binaries: (NH2)(CH3)C(CH2OH)2–(CH3)2C(CH2OH)2 system.
Calphad,
Vol. 27,
Issue. 2,
p.
133.
Witusiewicz, V.T.
Sturz, L.
Hecht, U.
and
Rex, S.
2004.
Thermodynamic description and unidirectional solidification of eutectic organic alloys: II. (CH3)2C(CH2OH)2–(NH2)(CH3)C(CH2OH)2 system.
Acta Materialia,
Vol. 52,
Issue. 17,
p.
5071.
Chellappa, Raja
Russell, Renee
and
Chandra, Dhanesh
2004.
Thermodynamic modeling of the C(CH2OH)4–(NH2)(CH3)C(CH2OH)2 binary system.
Calphad,
Vol. 28,
Issue. 1,
p.
3.
Divi, Suresh
Chellappa, Raja
and
Chandra, Dhanesh
2006.
Heat capacity measurement of organic thermal energy storage materials.
The Journal of Chemical Thermodynamics,
Vol. 38,
Issue. 11,
p.
1312.
Mishra, A.
Talekar, A.
Chandra, D.
and
Chien, W.-M.
2012.
Ternary phase diagram calculations of pentaerythritol–pentaglycerine–neopentylglycol system.
Thermochimica Acta,
Vol. 535,
Issue. ,
p.
17.
Mishra, Amrita
Talekar, Anjali
Shi, Renhai
and
Chandra, Dhanesh
2014.
Thermodynamic assessment of orientationally disordered organic molecular crystals: Ternary system pentaerythritol–neopentylglycol–2-amino-2methyl-1,3, propanediol (PE–NPG–AMPL).
Calphad,
Vol. 46,
Issue. ,
p.
108.
Singh, Harpreet
Talekar, Anjali
Chien, Wen-Ming
Shi, Renhai
Chandra, Dhanesh
Mishra, Amrita
Tirumala, Muralidhar
and
Nelson, Daryl J.
2015.
Continuous solid-state phase transitions in energy storage materials with orientational disorder – Computational and experimental approach.
Energy,
Vol. 91,
Issue. ,
p.
334.
Mekala, Prathyusha
Kamisetty, Vamsi
Chien, Wen-Ming
Shi, Renhai
Chandra, Dhanesh
Sangwai, Jitendra
Talekar, Anjali
and
Mishra, Amrita
2015.
Thermodynamic modeling of binary phase diagram of 2-amino-2-methyl-1, 3-propanediol and TRIS(hydroxymethyl)aminomethane system with experimental verification.
Calphad,
Vol. 50,
Issue. ,
p.
126.
Wang, Chao
Li, Qifeng
Wang, Liping
and
Lan, Xiaozheng
2016.
Phase transition of neopentyl glycol in nanopores for thermal energy storage.
Thermochimica Acta,
Vol. 632,
Issue. ,
p.
10.
Serrano, Angel
Dauvergne, Jean-Luc
Doppiu, Stefania
and
Palomo Del Barrio, Elena
2019.
Neopentyl Glycol as Active Supporting Media in Shape-Stabilized PCMs.
Materials,
Vol. 12,
Issue. 19,
p.
3169.
Li, Bing
Kawakita, Yukinobu
Ohira-Kawamura, Seiko
Sugahara, Takeshi
Wang, Hui
Wang, Jingfan
Chen, Yanna
Kawaguchi, Saori I.
Kawaguchi, Shogo
Ohara, Koji
Li, Kuo
Yu, Dehong
Mole, Richard
Hattori, Takanori
Kikuchi, Tatsuya
Yano, Shin-ichiro
Zhang, Zhao
Zhang, Zhe
Ren, Weijun
Lin, Shangchao
Sakata, Osami
Nakajima, Kenji
and
Zhang, Zhidong
2019.
Colossal barocaloric effects in plastic crystals.
Nature,
Vol. 567,
Issue. 7749,
p.
506.
Midhun, V. C.
Suresh, S.
Praveen, B.
and
Shiju, R. S.
2020.
Experimental study on phase transition behaviour of shape stable phase change material for application in vacuum insulation panel.
Journal of Energy Storage,
Vol. 32,
Issue. ,
p.
101825.
Cardona-Castro, M. A.
Leon-Gil, J. A.
and
Alvarez-Quintana, J.
2021.
A novel enhanced performance thermal rectifier based on NPG functionalized carbon fibers.
Materials Advances,
Vol. 2,
Issue. 18,
p.
5942.
Pan, Hailong
Luo, Jiangshui
Li, Bing
and
Wübbenhorst, Michael
2021.
Phase-dependent dielectric properties and proton conduction of neopentyl glycol.
RSC Advances,
Vol. 11,
Issue. 38,
p.
23228.
Pratap Singh, Ayush
Khanna, Sakshum
Paneliya, Sagar
Hinsu, Harsh
Patel, Yashkumar
and
Mehta, Bhashin
2021.
Preparation and characterization of solid-state neopentyl glycol / expanded graphite micro composite for thermal energy storage applications.
Materials Today: Proceedings,
Vol. 47,
Issue. ,
p.
621.
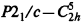 , space group No. 14), a = 5.979(1)Å, b= 10.876(2)Å, c=10.099(2)Å, β=99.78(1)°, V=647.2(2)Å3 at 20°(± 1)C, Dx= 1.069 g cm s−3 for Z=4. In this paper the redetermined structure of the α phase of NPG is presented in projections of the atomic positions, in tables, and in calculated powder pattern and these results are compared with those reported by others. The powder patterns obtained from the Bragg–Brentano diffractometer are compared with our calculated pattern from the single crystal data. The structural parameters of the high temperature phase of NPG as determined by a Guinier diffraction system are also reported.
, space group No. 14), a = 5.979(1)Å, b= 10.876(2)Å, c=10.099(2)Å, β=99.78(1)°, V=647.2(2)Å3 at 20°(± 1)C, Dx= 1.069 g cm s−3 for Z=4. In this paper the redetermined structure of the α phase of NPG is presented in projections of the atomic positions, in tables, and in calculated powder pattern and these results are compared with those reported by others. The powder patterns obtained from the Bragg–Brentano diffractometer are compared with our calculated pattern from the single crystal data. The structural parameters of the high temperature phase of NPG as determined by a Guinier diffraction system are also reported.

