Published online by Cambridge University Press: 25 August 2021
A ternary compound Al3CoNd2 was synthesized and its crystal structure parameters were determined by the Rietveld refinement method based on powder X-ray diffraction data. Results show that the compound crystallizes in the MgCu2-type structure (cubic Laves C15 phase, space group 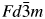 $Fd\bar{3}m$), with the lattice parameter of a = 7.8424(2) Ǻ, unit-cell volume of V = 482.33 Å3, and calculated density of Dcalc = 5.90 g.cm−3. The residual factors converge to Rp = 0.1024 and Rwp = 0.1287. The reference intensity ratio value obtained experimentally is 3.03. Magnetic susceptibility measurements indicate an agreement with the Curie–Weiss law in the temperature range of 385–450 K, and paramagnetic Curie temperature of θp = 379.9 K. Both rare-earth elements and cobalt ions contribute to the paramagnetic moment. The saturation magnetic moment and magnetic hysteresis loop were measured for the Al3CoNd2 compound at various temperatures. Results show that the saturation magnetic moment value decreases with an increase in temperature and the compound becomes a ferromagnet below the Curie temperature Tc.
$Fd\bar{3}m$), with the lattice parameter of a = 7.8424(2) Ǻ, unit-cell volume of V = 482.33 Å3, and calculated density of Dcalc = 5.90 g.cm−3. The residual factors converge to Rp = 0.1024 and Rwp = 0.1287. The reference intensity ratio value obtained experimentally is 3.03. Magnetic susceptibility measurements indicate an agreement with the Curie–Weiss law in the temperature range of 385–450 K, and paramagnetic Curie temperature of θp = 379.9 K. Both rare-earth elements and cobalt ions contribute to the paramagnetic moment. The saturation magnetic moment and magnetic hysteresis loop were measured for the Al3CoNd2 compound at various temperatures. Results show that the saturation magnetic moment value decreases with an increase in temperature and the compound becomes a ferromagnet below the Curie temperature Tc.