I. INTRODUCTION
Hydrochlorothiazide (HCTZ), shown in Figure 1, is a diuretic that is widely used in antihypertensive therapies.

Figure 1. Molecular structure of hydrochlorothiazide.
HCTZ exhibits two crystal structures known as Forms I and II. Form I (Space Group: P21) is known to have been reported by L. Dupont and O. Dideberg (CSD [Structural Database. Established in 1965, the CSD is the world's repository for small-molecule organic crystal structures]: HCSBTZ) (Dupont and Dideberg, Reference Dupont and Dideberg1972) while Form II (Space Group: P21/c) was reported in the work of A. Florence et al. (CSD: HCSBTZ01) (Florence et al., Reference Florence, Johnston, Fernandes, Shankland, Stevens, Osmundsen and Mullen2005).
According to Johnston et al., Reference Johnston, Florence, Shankland, Kennedy, Shankland and Price2007, Form II is said to be the metastable one, whereas Form I, which corresponds to the commercial form, is known to be the one with the highest stability. (Johnston et al., Reference Johnston, Florence, Shankland, Kennedy, Shankland and Price2007).
The polymorphic characterization can be developed by several techniques, among them including spectroscopic (IR, Raman, NMR) (Brittain, Reference Brittain1997; Guo et al.,) (Brittain, Reference Brittain1997; Linck et al.,), thermal analysis (TG, DTA, DSC), and microscopic (SEM, TEM, FEG). Another widely applied technique is X-ray powder diffraction (XRPD). Largely used in solid-state characterization, the XRPD when combined with the Rietveld Method (RM) (Rietveld, Reference Rietveld1969), is remarkably an interesting tool used for the characterization as well as the quantification of crystalline phases.
Manufacturing processes such as milling, recrystallization, drying, etc. may change the powder properties and are likely to exert an influence over the stability and the dissolution of a solid pharmaceutical formulation.
In the present study, the polymorphic form of HCTZ was investigated in commercial formulations of HCTZ using the RM and XRPD data. The excipients of these tablets were also characterized.
II. EXPERIMENTAL
Commercial tablets were purchased from some drugstores located in the city of Araraquara (São Paulo State, Brazil). The medicaments acquired included a reference one (sample R) and two generics (samples G-01 and G-02, respectively), all the formulations were of 25 mg. The coating of the tablets was removed and the tablets were then ground and sieved, the fine powder was pressed in a flat-plate sample holder. The data were collected using a Rigaku RINT2000 cooper (CuKα) rotating anode diffractometer operating at 42 kV and 120 mA. A receiving slit of 0.3 mm, a 2.5° divergence Soller slit and curved graphite monochromator in the diffracted beam were used. The data were collected in the 2θ scan range 2°–40°, at room temperature. The refinements were performed using TOPAS Academic v.5 software (Coelho, Reference Coelho2007). A fundamental parameters model implemented in the program was used to adjust the peak broadening (Cheary et al., Reference Cheary, Coelho and Cline2004). The crystallite size was refined considering an anisotropic form, having independent Lorentzian and Gaussian components to the family plans (h00), (0k0), (00l), (hk0), (h0l), and (hkl), an approximation of the Double–Voigt model which is implemented in the software TOPAS Academic v.5 (Balzar, Reference Balzar1993). A Chebyschev polynomial was used to adjust the background (Chebyshev, Reference Chebyshev1854). The preferred orientation feature was corrected using the Spherical Harmonics model (Järvinen, Reference Järvinen1993). The unit-cell parameters of HCTZ Form I and excipients were refined, but the atomic coordinates of the crystal phases were not.
III. RESULTS AND DISCUSSIONS
The information on the package insert of the medicaments is in Table I, where the excipients presented in each formulation can clearly be found. The crystal structure of HCTZ Form I was employed in the refinement of the three samples, which does not present the peaks of Form II of this drug.
Table I. Package insert information for the three medicaments analyzed; samples R (the reference medicament), G-01, and G-02 (two generics).
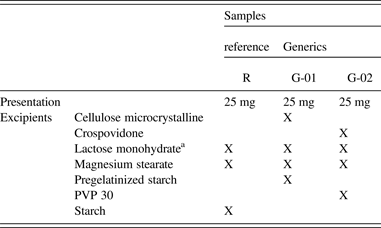
aThis crystal phase was identified in the refinement.
Lactose monohydrate is an excipient which is largely used in tablets production, and it is usually found in the crystalline form. The X-ray powder pattern of lactose monohydrate (space group: P21) (Fries et al., Reference Fries, Rao and Sundaralingam1971) was found in the tablets, and its crystal structure model was employed in the refinement.
Table II shows the cell parameters of the crystal structures that were employed in the refinements. It is worth noting that the sodium lauril sulfate (SLS) does not belong to the package insert of the medicament, but its characteristic peaks are present in the diffractogram of the G-02 sample, which was characterized during the refinement.
Table II. Cell parameters of the crystal structures employed in the refinements.

aLM, lactose monohydrate.
bSLS, sodium lauryl sulfate.
A. Refinements
1. Sample R
The reference tablet, sample R, presented Form I of HCTZ and the peaks of a crystalline lactose monohydrate excipient. The Rietveld plot of this sample is shown in Figure 2, where the adequate agreement of the profile fitting can be observed. The Rietveld plot shows the observed powder pattern (◦), the calculated pattern (continuous line), the difference between the calculated and the observed patterns, and the Bragg peak positions (|). This tablet contains other excipients, as can be observed in Table I, though they do not exhibit peaks. This sample contains amorphous starch, cellulose microcrystalline, crospovidone, and polyvinylpyrrolidone (PVP). In the package insert, it can be found that the excipient magnesium stearate is cited. This excipient is normally crystalline and tend to exhibit its most intense peak at 2θ ~ 5.5°. Though, no peak of this excipient was observed in this position in the diffractogram. The quantitative phase analysis from the RM only quantifies crystalline phases and, in this case, there are some amorphous phases of the above-mentioned excipients. Thus, the results do not represent all the components of the tablet but only the crystalline phases. Sample R exhibited 14.4(1) wt% of active principle ingredient (API) and 85.6(1) wt% of lactose monohydrate.

Figure 2. (Color online) Rietveld plot of the reference sample, showing Form I of HCTZ and the crystalline excipient lactose monohydrate.
2. Sample G-01
The Rietveld plot of the G-01 sample is shown in Figure 3, where the peaks of Form I of HCTZ, the crystalline excipient lactose monohydrate and the magnesium stearate can all be observed. The quantitative phase analyses for the sample exhibited 29.2(2) wt% of API and 70.8(2) wt% of lactose monohydrate. As the magnesium stearate do not have the crystal structure, it was possible to identify but not quantify.

Figure 3. (Color online) Rietveld plot of the generic G-01, showing Form I of HCTZ and the crystalline excipient lactose monohydrate.
3. Sample G-02
The other generic medicament – sample G-02, has its Rietveld plot shown in Figure 4. The peaks of Form I of HCTZ, the crystalline excipient lactose monohydrate, magnesium stearate, and SLS have all been identified though the latter was not mentioned in the package insert of the medicament. Owing to the fact that the characteristic peaks of SLS (2.68° and 4.49°) do not overlap with the peaks of any other excipients, it was considered present in the tablet, and hence included in the Rietveld refinement. The quantitative phase analyses for the sample exhibited 23.1 (2) wt% of API, 76.6(2) wt% of lactose monohydrate, and 0.38 (2) wt% of SLS. The quantification of SLS was possible because its main peak [2.2°, which corresponds to (100)] appeared in a diffraction region without overlapping with other crystalline phases.

Figure 4. (Color online) Rietveld plot of the sample G-02, showing Form I of HCTZ, the excipient lactose monohydrate (L.M), the magnesium stearate (Mg.S.) and the excipient not mentioned in the package insert: sodium lauryl sulfate (SLS), where the most intense peaks are indicated in the insert.
The refinements agreements factor of the Rietveld refinements are given in Table III. The cell parameters were refined to adjust the peak position and the values obtained after the adjustment can also be found in Table III. The cell parameter values, following refinements, have remained close to the values of the crystallographic information framework (CIF) that was used.
Table III. Agreement factors and cell parameters obtained from the refinements of the samples R, G-01, and G-02.

aHCTZ, hydrochlorothiazide.
bLM, lactose monohydrate.
cSLS, sodium lauryl sulfate.
The quantitative phase analysis of API of the three samples show significant differences (Sample R 14.4; Sample G-01 29.2; Sample G-02 23.1), indicating that among these formulations differences can be found in the quantity of amorphous excipients.
IV. CONCLUSIONS
All the tablets (the reference one and the two generic formulations of 25 mg) exhibited Form I of HCTZ, which is the commercial form of this API.
All samples exhibited the crystal structure of the lactose monohydrate, a crystalline excipient, which peaks have been characterized during the refinements. The generic medicament G-02 exhibited the characteristic peaks of SLS, a crystalline excipient which has not been declared in the package insert of this medicament.
Acknowledgments
The authors express their gratitude for the work supported by Capes and CNPq, and to FAPESP, a São Paulo State Research Foundation in Brazil.










