Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Fortes, A. Dominic
Browning, Frank
and
Wood, Ian G.
2012.
Cation substitution in synthetic meridianiite (MgSO4·11H2O) I: X-ray powder diffraction analysis of quenched polycrystalline aggregates.
Physics and Chemistry of Minerals,
Vol. 39,
Issue. 5,
p.
419.
Fortes, A. Dominic
Wood, Ian G.
and
Gutmann, Matthias J.
2013.
MgSO4·11H2O and MgCrO4·11H2O based on time-of-flight neutron single-crystal Laue data.
Acta Crystallographica Section C Crystal Structure Communications,
Vol. 69,
Issue. 4,
p.
324.
Fortes, A. Dominic
2015.
X-ray powder diffraction analysis of two new magnesium selenate hydrates, MgSeO4·9H2O and MgSeO4·11H2O.
Powder Diffraction,
Vol. 30,
Issue. 2,
p.
149.
Fortes, A. Dominic
Alfè, Dario
Hernández, Eduardo R.
and
Gutmann, Matthias J.
2015.
Structure of magnesium selenate enneahydrate, MgSeO4·9H2O, from 5 to 250 K using neutron time-of-flight Laue diffraction.
Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials,
Vol. 71,
Issue. 3,
p.
313.
Fortes, A.D.
Wood, I.G.
Hudson-Edwards, K.A.
and
Gutmann, M.J.
2017.
Partitioning of Co2+ and Mn2+ into meridianiite (MgSO4·11H2O): Ternary solubility diagrams at 270 K; cation site distribution determined by single-crystal time-of-flight neutron diffraction and density functional theory.
Fluid Phase Equilibria,
Vol. 437,
Issue. ,
p.
1.
Nowak, Przemysław
Pietrzak, Anna
and
Dudek, Marta K.
2025.
Understanding the stability of the amorphous form of darifenacin.
CrystEngComm,
Vol. 27,
Issue. 13,
p.
1927.
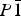 , Z = 2, with unit-cell parameters a = 6.811 33(8) Å, b = 6.958 39(9) Å, c = 17.3850(2) Å, α = 87.920(1)°, β = 89.480(1)°, γ = 62.772(1)°, and V = 732.17(1) Å3 at −15 °C. The difference in unit-cell parameters between SO4- and CrO4-bearing species is only partially accounted for by the difference in S–O and Cr–O bond lengths; the remainder of the difference (over 90% in the cell volume) is attributed to weakening of the interpolyhedral hydrogen-bond network.
, Z = 2, with unit-cell parameters a = 6.811 33(8) Å, b = 6.958 39(9) Å, c = 17.3850(2) Å, α = 87.920(1)°, β = 89.480(1)°, γ = 62.772(1)°, and V = 732.17(1) Å3 at −15 °C. The difference in unit-cell parameters between SO4- and CrO4-bearing species is only partially accounted for by the difference in S–O and Cr–O bond lengths; the remainder of the difference (over 90% in the cell volume) is attributed to weakening of the interpolyhedral hydrogen-bond network.