Published online by Cambridge University Press: 13 March 2013
The structures of γ- and δ-K4P2O7 are solved by X-ray powder diffraction (conventional laboratory X-ray and synchrotron data, respectively), both in hexagonal symmetry (aγ = 5.9645(3) Å, cγ = 14.4972(8) Å, Vγ = 446.64(4) Å3 at 300 °C, Zγ = 2, space group P63/mmc; aδ = 10.211 45(7) Å, cδ = 42.6958(4) Å, Vδ = 3855.59(7) Å3 at room temperature, Zδ = 18, space group P61) with cell–supercell relations 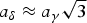 $a_\delta \approx a_\gamma \sqrt{3}$ and cδ ≈ 3 cγ. In the experimental conditions, the expected β/γ transition previously announced at 486 °C is not observed; the γ-form is stable at least up to the maximum temperature of our measurements (700 °C). In the γ-form, similar to the orthorhombic form of Na4P2O7, idealized, the pyrophosphate group is in eclipsed conformation, the K+ cations occupying three different coordinations. In the δδ-form, two of the three different [P2O7]4− groups are staggered and one eclipsed, the K+ cations occupying 12 independent sites.
$a_\delta \approx a_\gamma \sqrt{3}$ and cδ ≈ 3 cγ. In the experimental conditions, the expected β/γ transition previously announced at 486 °C is not observed; the γ-form is stable at least up to the maximum temperature of our measurements (700 °C). In the γ-form, similar to the orthorhombic form of Na4P2O7, idealized, the pyrophosphate group is in eclipsed conformation, the K+ cations occupying three different coordinations. In the δδ-form, two of the three different [P2O7]4− groups are staggered and one eclipsed, the K+ cations occupying 12 independent sites.