Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Fong, C.
Kennedy, B. J.
and
Elcombe, M. M.
1994.
A powder neutron diffraction study of λ and γ manganese dioxide and of LiMn2O4
.
Zeitschrift für Kristallographie - Crystalline Materials,
Vol. 209,
Issue. 12,
p.
941.
Kennedy, Brendan J.
Hunter, Brett A.
and
Howard, Christopher J.
1997.
Structural and Bonding Trends in Tin Pyrochlore Oxides.
Journal of Solid State Chemistry,
Vol. 130,
Issue. 1,
p.
58.
Ismunandar
Kennedy, Brendan J.
Hunter, Brett A.
and
Vogt, Tom
1997.
Bonding and Structural Variations in Doped Bi2Sn2O7.
Journal of Solid State Chemistry,
Vol. 131,
Issue. 2,
p.
317.
Ismunandar
Kennedy, Brendan J.
and
Hunter, Brett A.
1999.
Observations on pyrochlore oxide structures.
Materials Research Bulletin,
Vol. 34,
Issue. 8,
p.
1263.
Rammutla, K E
Chadwick, A V
Harding, J
Sayle, D C
and
Erasmus, R M
2010.
EXAFS and Raman scattering studies of Y and Zr doped nano-crystalline tin oxide.
Journal of Physics: Conference Series,
Vol. 249,
Issue. ,
p.
012054.
Mouta, R.
Silva, R. X.
and
Paschoal, C. W. A.
2013.
Tolerance factor for pyrochlores and related structures.
Acta Crystallographica Section B Structural Science Crystal Engineering and Materials,
Vol. 69,
Issue. 5,
p.
439.
Ntimane, N. J.
Rammutla, K. E.
and
Mosuang, T. E.
2015.
Effects of combinational Al and Y doping on the structural and optical properties of nanocrystalline SnO2.
Indian Journal of Physics,
Vol. 89,
Issue. 7,
p.
671.
Fan, Wenjie
Hu, Jinli
Huang, Jing
Wu, Xin
Lin, Sen
Huang, Caijin
and
Qiu, Xiaoqing
2015.
Electronic structure and photocatalytic activities of (Bi2−Y )Sn2O7 solid solution.
Applied Surface Science,
Vol. 357,
Issue. ,
p.
2364.
Brik, M.G.
Srivastava, A.M.
Ma, C.-G.
and
Piasecki, M.
2020.
Dependence of the Mn4+ spectroscopic properties on the host composition: Case study of stannate pyrochlores A2Sn2O7 (A = La, Gd, Y, Lu).
Journal of Luminescence,
Vol. 218,
Issue. ,
p.
116834.
Hensling, Felix V. E.
Dahliah, Diana
Dulal, Prabin
Singleton, Patrick
Sun, Jiaxin
Schubert, Jürgen
Paik, Hanjong
Subedi, Indra
Subedi, Biwas
Rignanese, Gian-Marco
Podraza, Nikolas J.
Hautier, Geoffroy
and
Schlom, Darrell G.
2021.
Epitaxial stannate pyrochlore thin films: Limitations of cation stoichiometry and electron doping.
APL Materials,
Vol. 9,
Issue. 5,
Converse, Elizabeth Sobalvarro
King, Gabriella
Li, Jun
and
Subramanian, M.A.
2022.
Bond valence sum analysis of pyrochlore oxides including the novel dielectric Te6+ pyrochlores: ABiMTeO7- (A = Cd, Ca; M = Cr, Ga, Sc, In, Fe).
Progress in Solid State Chemistry,
Vol. 68,
Issue. ,
p.
100378.
Borah, Kulendra
Longchar, Lanuakum A.
Babu, P.D.
Jayaramulu, Kolleboyina
and
Sundarayya, Y.
2024.
Surface Ferromagnetism in Y2Sn2O7 Pyrochlore Nanoparticles.
Journal of Alloys and Compounds,
Vol. 1008,
Issue. ,
p.
176662.
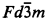 with a = 10.3723(1) Å, Dx = 6.21 g cm−3, and with the oxygen position parameter of 0.33694(5). The Sn atoms are in a nearly regular octahedral coordination whereas the Y has a distorted 8-fold coordination geometry. The anisotropic thermal parameters were also determined. The refined model has been used to calculate a set of d-I X-ray data for search/match analysis.
with a = 10.3723(1) Å, Dx = 6.21 g cm−3, and with the oxygen position parameter of 0.33694(5). The Sn atoms are in a nearly regular octahedral coordination whereas the Y has a distorted 8-fold coordination geometry. The anisotropic thermal parameters were also determined. The refined model has been used to calculate a set of d-I X-ray data for search/match analysis.

