Published online by Cambridge University Press: 02 May 2017
Structures of the two M0.50SbFe(PO4)3 (M = Mg, Ni) phases, abbreviated as [Mg0.50] and [Ni0.50], were determined at room temperature from X-ray diffraction (XRD) powder data using the Rietveld analysis. Both compounds belong to the NASICON structural family. XRD patterns of [Mg0.50] and [Ni0.50] phases were easily indexed with a primitive hexagonal unit cell [P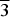 $\overline 3 $ space group, Z = 6] similar to that already obtained for La0.33Zr2(PO4)3. Obtained unit cells parameters are [a = 8.3443(1) Å, c = 22.3629(1) Å], and [a = 8.3384(1), c = 22.3456(1) Å], respectively, for [Mg0.50] and [Ni0.50] phosphates. In both samples, the [Sb(Fe)(PO4)3]− NASICON framework is preserved and a partially-ordered distribution of Sb5+ and Fe3+ ions is observed. Raman spectroscopic study was used to obtain further structural information about the nature of bonding in [Mg0.50] and [Ni0.50] phases.
$\overline 3 $ space group, Z = 6] similar to that already obtained for La0.33Zr2(PO4)3. Obtained unit cells parameters are [a = 8.3443(1) Å, c = 22.3629(1) Å], and [a = 8.3384(1), c = 22.3456(1) Å], respectively, for [Mg0.50] and [Ni0.50] phosphates. In both samples, the [Sb(Fe)(PO4)3]− NASICON framework is preserved and a partially-ordered distribution of Sb5+ and Fe3+ ions is observed. Raman spectroscopic study was used to obtain further structural information about the nature of bonding in [Mg0.50] and [Ni0.50] phases.