Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Facio, Jorge I.
Das, Sanjib Kumar
Zhang, Yang
Koepernik, Klaus
van den Brink, Jeroen
and
Fulga, Ion Cosma
2019.
Dual topology in jacutingaite
Pt2HgSe3.
Physical Review Materials,
Vol. 3,
Issue. 7,
Marrazzo, Antimo
Gibertini, Marco
Campi, Davide
Mounet, Nicolas
and
Marzari, Nicola
2019.
Relative Abundance of Z2 Topological Order in Exfoliable Two-Dimensional Insulators.
Nano Letters,
Vol. 19,
Issue. 12,
p.
8431.
de Lima, F. Crasto
Miwa, R. H.
and
Fazzio, A.
2020.
Jacutingaite-family: A class of topological materials.
Physical Review B,
Vol. 102,
Issue. 23,
Ma, Chi
Förster, Hans-Jürgen
and
Grundmann, Günter
2020.
Tilkerodeite, Pd2HgSe3, a New Platinum-Group Mineral from Tilkerode, Harz Mountains, Germany.
Crystals,
Vol. 10,
Issue. 8,
p.
687.
Longuinhos, R
Vymazalová, A
Cabral, A R
and
Ribeiro-Soares, J
2021.
Raman spectrum of layered tilkerodeite (Pd2HgSe3) topological insulator: the palladium analogue of jacutingaite (Pt2HgSe3).
Journal of Physics: Condensed Matter,
Vol. 33,
Issue. 6,
p.
065401.
Longuinhos Monteiro Lobato, Raphael
and
Ribeiro-Soares, Jenaina
2021.
Mechanical properties of layered tilkerodeite (Pd2HgSe3) and jacutingaite (Pt2HgSe3) crystals: Insights on the interlayer, intralayer interactions, and phonons.
Journal of Applied Physics,
Vol. 130,
Issue. 1,
Hajati, Yaser
Alipourzadeh, Mohammad
Makhfudz, Imam
and
Berakdar, Jamal
2025.
Electromagnetically tunable spin-valley-polarized current via anomalous Nernst effect in monolayer of jacutingaite.
Journal of Physics: Condensed Matter,
Vol. 37,
Issue. 6,
p.
065802.
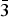 $\bar 3$m1 space group with the unit-cell parameters a = 7.3096(2) Å, c = 5.2829(1) Å, V = 244.45(1) Å3, Dc = 8.84 g/cm3, and Z = 2. In its layered crystal structure, the [PdSe6] octahedra share opposing Se–Se edges with adjacent [PdSe4] squares forming layers parallel with the (001) plane. The layers show AA type stacking along the c-axis. Hg atoms occupy the anti-cubooctahedral voids between two consecutive layers. Pd2HgSe3 is isostructural with Pt2HgSe3 and Pt4Tl2X6 (X = S, Se, or Te) phases. The structure can be viewed as a 2a.2a.c superstructure of PtSe2.
$\bar 3$m1 space group with the unit-cell parameters a = 7.3096(2) Å, c = 5.2829(1) Å, V = 244.45(1) Å3, Dc = 8.84 g/cm3, and Z = 2. In its layered crystal structure, the [PdSe6] octahedra share opposing Se–Se edges with adjacent [PdSe4] squares forming layers parallel with the (001) plane. The layers show AA type stacking along the c-axis. Hg atoms occupy the anti-cubooctahedral voids between two consecutive layers. Pd2HgSe3 is isostructural with Pt2HgSe3 and Pt4Tl2X6 (X = S, Se, or Te) phases. The structure can be viewed as a 2a.2a.c superstructure of PtSe2.