Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kotova, Irina Yu.
Savina, Aleksandra A.
Vandysheva, Alena I.
Belov, Dmitry A.
and
Stefanovich, Sergey Yu.
2018.
Synthesis, crystal structure and electrophysical properties of triple molybdates containing silver, gallium and divalent metals.
Chimica Techno Acta,
Vol. 5,
Issue. 3,
p.
132.
Aksenov, Sergey M.
Pavlova, Erzhena T.
Popova, Nadezhda N.
Tsyrenova, Galina D.
and
Lazoryak, Bogdan I.
2024.
Stoichiometry and topological features of triple molybdates ABC(MoO4) with the heteropolyhedral open MT-frameworks: Synthesis, crystal structure of Rb5{Hf1.5Co0.5(MoO4)6}, and comparative crystal chemistry.
Solid State Sciences,
Vol. 151,
Issue. ,
p.
107525.
Wang, Y. P.
Yang, W. B.
Ling, S.
Xiong, F. B.
and
Ma, E.
2024.
Effect of substituting Y3+/W6+/F− ions on the luminescent properties of novel red-emitting NaMg3In(MoO4)5: Eu3+ phosphors for white LED.
Journal of Materials Science: Materials in Electronics,
Vol. 35,
Issue. 8,
Kotova, Irina
Spiridonova, Tatyana
Savina, Alexandra
Khaikina, Elena
Solodovnikov, Sergey
Solodovnikova, Zoya
Koo, Hyun-Joo
Whangbo, Myung-Hwan
Ovchenkov, Yevgeniy
Zakharov, Konstantin
Vasilchikova, Tatyana
Shvanskaya, Larisa
and
Vasiliev, Alexander
2025.
Crystal structure and ferrimagnetism of AgCo3Cr(MoO4)5 with mixed occupation of the transition metal sites.
Dalton Transactions,
Vol. 54,
Issue. 12,
p.
5128.
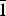 $\bar 1$). In the structure pairs of edge-shared (Mg, Ga)O6, octahedra are connected by common vertices to form a three-dimensional framework. Large framework cavities involve Ag+ cations. The title compound was found to melt at 1079 K.
$\bar 1$). In the structure pairs of edge-shared (Mg, Ga)O6, octahedra are connected by common vertices to form a three-dimensional framework. Large framework cavities involve Ag+ cations. The title compound was found to melt at 1079 K.