Introduction
Prior to the 2020 Presidential Election, TheUpshot blog for the New York Times queried if readers could correctly discern from a photo whether the contents of a refrigerator belonged to a Republican or a Democrat (Keefe, Reference Keefe2020). While some refrigerators were correctly ascribed to a party affiliation more than 80% of the time, the upshot of hundreds of thousands of guesses was that they were accurate a paltry 52% of the time. This is close to a coin toss. Though many aspects of American life and culture are politically polarized, including restaurants and grocery store chains (Wasserman, Reference Wasserman2014), the foods that end up in our kitchens do not seem to be.
This project, however, asked whether the mental processes underlying food choice differ by political party, while we confirmed that the actual choices do not. Because the brain performs uncountable related and unrelated actions using the same neural circuitry, it follows that areas of the brain used for one set of decisions are probably going to be used for other decisions. The growing body of decision-neuroscience, neuroeconomics, and neuropolitics literature supports this (Boyland et al., Reference Boyland, Maden, Coates, Masterson, Alblas, Bruce and Roberts2024; Dennison et al., Reference Dennison, Sazhin and Smith2022). Fundamental work in neuroscience has shown that animals with different neural circuitry and activations may still engage in the same behaviors and make the same decisions (Marder, Reference Marder2011).
This study examines if the political affiliation of adults who self-identify as Democrat or Republican differs based on brain activity during a decision unrelated to politics. Using two experiments on food purchases, we show that political parties can be relatively well-differentiated not because of the actual foods purchased but because of brain activity when making those purchases. Brain activations in five brain regions diverge by political affiliation during a food purchase. Even though the foods the subjects chose do not differ by party affiliation, the brain activity differences are significant enough to allow us to correctly classify consumers as Republicans or Democrats.
Neuroimaging techniques have allowed scientists to explore brain differences between adults identifying as Republicans and Democrats or conservatives and liberals in socio-political experiments, including face judgment, partisanship, motivated reasoning, political interest, political attitudes, and automatic processing of political preferences (Krastev et al., Reference Krastev, McGuire, McNeney, Kable, Stolle, Gidengil and Fellows2016). For example, Schreiber et al. (Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013) show that the brain’s evaluation processes in a non-social, non-political, risk-taking experiment are distinct between Republicans and Democrats. Yet, what about day-to-day decisions? Will brain differences that predict political affiliation still exist if the experimental stimulus is something as simple as an apolitical, single-item food purchase?
The field of decision neuroscience has grown recently, with some common brain areas frequently identified to be fundamental for making choices about money, food, and political preferences (Figure 1). The brain regions commonly documented to be associated with political attitudes and behavior are emotional regions, including the amygdala (Gozzi et al., Reference Gozzi, Zamboni, Krueger and Grafman2010; Kanai et al., Reference Kanai, Feilden, Firth and Rees2011; Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006; Petalas et al., Reference Petalas, Schumacher and Scholte2024; Rule et al., Reference Rule, Freeman, Moran, Gabrieli, Adams and Ambady2010), insular cortex (Kaplan et al., Reference Kaplan, Freedman and Iacoboni2007; Krosch et al., Reference Krosch, Jost and Van Bavel2021; Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013; Westen et al., Reference Westen, Blagov, Harenski, Kilts and Hamann2006), anterior cingulate cortex (Amodio et al., Reference Amodio, Jost, Master and Yee2007; Kanai et al., Reference Kanai, Feilden, Firth and Rees2011; Kaplan et al., Reference Kaplan, Freedman and Iacoboni2007; Westen et al., Reference Westen, Blagov, Harenski, Kilts and Hamann2006), ventromedial prefrontal cortex (vmPFC)(Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006; Mitchell et al., Reference Mitchell, Macrae and Banaji2006; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009), dorsomedial prefrontal cortex (dmPFC)(Mitchell et al., Reference Mitchell, Macrae and Banaji2006; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009), dorsolateral prefrontal cortex (dlPFC)(Kaplan et al., Reference Kaplan, Freedman and Iacoboni2007; Kato et al., Reference Kato, Ide, Kabashima, Kadota, Takano and Kansaku2009; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009), ventral striatum (Gozzi et al., Reference Gozzi, Zamboni, Krueger and Grafman2010; Tusche et al., Reference Tusche, Kahnt, Wisniewski and Haynes2013; Westen et al., Reference Westen, Blagov, Harenski, Kilts and Hamann2006; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009), and precuneus (Fowler & Schreiber, Reference Fowler and Schreiber2008; Gordon et al., Reference Gordon, Quadflieg, Brooks, Ecker and Lewandowsky2019; Kaplan et al., Reference Kaplan, Gimbel and Harris2016; Moore et al., Reference Moore, Hong and Cram2021). Tusche et al. (Reference Tusche, Kahnt, Wisniewski and Haynes2013) suggest that partisan bias may operate even in the absence of explicit attention to political content, yet few studies have examined the link between political ideology, brain activity, and non-political content in experiments.

Figure 1. Brain regions commonly activated during decision-making tasks.
When looking specifically at food, Hibbing et al. (Reference Hibbing, Smith and Alford2013) indicated that food preferences may reveal political preferences, Chuck, Fernandes & Hyers (Reference Chuck, Fernandes and Hyers2016) discuss how the politicization of diet can be part of one’s social identity and Lusk (Reference Lusk2012) shows that there are strong ideological leanings in support of or opposition to a host of food policies. Furthermore, Mosier & Rimal (Reference Mosier and Rimal2020) concluded that Democrats or non-affiliated individuals will report being vegan or vegetarian with a higher probability when compared to Republicans. Our interest is not related to self-reported behavior and revealed preferences for food but, as discussed in Sayre (Reference Sayre2011), how the underlying process of thinking about food reveals political identity.
To identify brain regions most likely to be implicated in decision-making around food, we conducted a meta-analysis of the impact of food advertising upon decision-making in adults and youth, merging data from neuroimaging studies of exposure to food marketing stimuli (versus control) on brain activations in children and adults to clarify relevant brain regions. Eleven studies met inclusion criteria; eight were used for this Activation Likelihood Estimation (ALE) meta-analysis (Eickhoff et al., Reference Eickhoff, Bzdok, Laird, Kurth and Fox2012). Food marketing exposures (versus controls) produced greater activation in two clusters lying across the middle occipital gyrus, lingual gyrus, and cuneus and postcentral gyrus, precentral gyrus, and the inferior parietal lobule/supramarginal gyrus. This meta-analysis demonstrated that brain responses to food advertising are observed in areas relating to visual processing, attention, sensorimotor activity, and emotional processing.
For this study, we examined two sets of healthy adult participants from the United States in separate experiments. One group made food purchase decisions about milk, and the other group made purchase decisions about eggs. The impetus for these food groups was that milk and egg products are so commonly purchased that consumers who purchase them likely have long-established preferences. While previous studies have demonstrated differential brain activation between partisans under conditions of threat, risk, uncertainty, or disgust, the present study demonstrates brain activity differs between Democrats and Republicans during a more mundane and less affectively charged non-political task, food purchasing.
Why brain activity during food purchase decisions might illuminate political identity
The ongoing debate about the nature and origin of mass opinion (Converse, Reference Converse and Apter1964; Zaller, Reference Zaller1992) is undergoing tremendous flux (Carmines & D’Amico, Reference Carmines and D’Amico2015). While there is some argument about the role of elite discourse (Fiorina & Abrams, Reference Fiorina and Abrams2009; Webster & Abramowitz, Reference Webster and Abramowitz2017), polarization (Barber & McCarty, Reference Barber, McCarty, Mansbridge and Martin2016), and affective partisanship (Iyengar et al., Reference Iyengar, Lelkes, Levendusky, Malhotra and Westwood2018; Mason, Reference Mason2018) as external or top-down influences, the role of internal or bottom-up influences is far more contested.
A wide variety of individual-level or psychological mechanisms have been proposed as explanatory factors in political ideology, including authoritarianism (Adorno, Reference Adorno1950; Feldman & Stenner, Reference Feldman and Stenner1997), social dominance orientation (Sidanius & Pratto, Reference Sidanius and Pratto1999), motivated social cognition (Jost & Amodio, Reference Jost and Amodio2012; Jost et al., Reference Jost, Glaser, Kruglanski and Sulloway2003), personality (Bakker & Lelkes, Reference Bakker and Lelkes2018; McClosky, Reference McClosky1958), moral foundations (Haidt & Graham, Reference Haidt and Graham2007), and values (Rokeach, Reference Rokeach1968; Sagiv et al., Reference Sagiv, Roccas, Cieciuch and Schwartz2017; Schwartz, Reference Schwartz and Zanna1992). However, it has often been extremely difficult to disentangle these factors from the political context, and in many cases, we find that “foundations” are not playing the role they were expected to play (Hatemi et al., Reference Hatemi, Crabtree and Smith2019; Hatemi & Verhulst, Reference Hatemi and Verhulst2015).
Heritable biological factors have been repeatedly shown to be correlated with political attitudes and behavior (Alford et al., Reference Alford, Funk and Hibbing2005; Hatemi & McDermott, Reference Hatemi and McDermott2012; Smith et al., Reference Smith, Alford, Hatemi, Eaves, Funk and Hibbing2012), however, the mediating factors are less clear (Hatemi & McDermott, Reference Hatemi and McDermott2016; Jost et al., Reference Jost, Nam, Amodio and Van Bavel2014). Initial reports suggested that biometric measures such as skin conductance levels could illuminate the relationship between biology and political attitudes (Oxley et al., Reference Oxley, Smith, Alford, Hibbing, Miller, Scalora and Hibbing2008). However, recent work has shown these findings do not replicate (Bakker et al., Reference Bakker, Schumacher, Gothreau and Arceneaux2020; Osmundsen et al., Reference Osmundsen, Hendry, Laustsen, Smith and Petersen2022). The authors of one of these failed replications “urge more, not less, research at the intersection of neuroscience and politics” (Bakker et al., Reference Bakker, Schumacher, Gothreau and Arceneaux2020, p. 5).
Brain imaging like functional magnetic resonance imaging (fMRI) may have a particular advantage in this context as fMRI has been shown to be a more powerful predictor of mass behavior beyond self-report than biometrics, implicit association tasks, eye tracking, or electroencephalography (Venkatraman et al., Reference Venkatraman, Dimoka and Pavlou2015). For instance, brain activity in response to disgusting images enables a highly accurate estimate of a participant’s political orientation, with even one single image being sufficient for correct classification (Ahn et al., Reference Ahn, Kishida, Gu, Lohrenz, Harvey, Alford and Montague2014). Activity associated with the amygdala, in particular, has been shown to differentiate liberals and conservatives as they make risky decisions (Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013) or experience the threat of physical pain (Pedersen et al., Reference Pedersen, Muftuler and Larson2018). The value of brain activity with nonpolitical stimuli as a correlate of political orientation is particularly intriguing and conceptually consistent with results demonstrating differences in brain structure correlating with political identity (Kanai et al., Reference Kanai, Feilden, Firth and Rees2011). The structural brain differences exist not only during moments of political activity; thus, it is reasonable that these differences may have implications in nonpolitical contexts.
These differences may be connected to biologically heritable factors, but the predictive power of the functional brain differences goes beyond what we would expect even if genetics were perfectly determining the differences we see in the brain (Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013). External factors familiar to traditional political science may be interacting with biological and other influences internal to the individual in order to generate our political attitudes, behaviors, and identities and also alter the structure and function of the brain (Hatemi & McDermott, Reference Hatemi and McDermott2016). The consequence, then, is not a causal story where genes and brains determine politics but rather a view of human nature where politics also shapes our biology (Fowler & Schreiber, Reference Fowler and Schreiber2008; Jost et al., Reference Jost, Nam, Amodio and Van Bavel2014).
Decisions about food provide a particularly fascinating case for investigating the possible interactions between politics and biology. Choices about what to eat are not only frequent but they are often tightly tied to identity, especially when those choices are costly (Henrich, Reference Henrich2009). In his book Collapse, Jared Diamond (Reference Diamond2005) cites the example of the Greenland Norse, who died out rather than eating the fish that comprised the diet of their Inuit neighbors. Samuel Popkin (Reference Popkin1991) contends that in the context of limited knowledge, voters will often rely on shortcuts in discerning whom to align with, highlighting Gerald Ford’s famous error of eating the corn husk around a tamale or George McGovern’s mistake of ordering milk with a kosher hot dog. Core values have been shown to be connected with both our food choices (Dreezens et al., Reference Dreezens, Martijn, Tenbült, Kok and de Vries2005) and our political decisions (Schwartz et al., Reference Schwartz, Caprara, Vecchione, Bain, Bianchi, Caprara and Zaleski2014). Preliminary work has tied both our food preferences and political preferences to heritable traits (Hibbing et al., Reference Hibbing, Smith and Alford2013).
Researchers have looked for political differences in measures of both odors (Friesen et al., Reference Friesen, Gruszczynski, Smith and Alford2020) and taste perception (Friesen et al., Reference Friesen, Ksiazkiewicz and Gothreau2021). Intriguingly, there is evidence that sexual mate sorting on ideology may be operating on olfactory cues (McDermott et al., Reference McDermott, Tingley and Hatemi2014). These smell and taste perceptions can also feed into our view that a particular stimulus is disgusting, with our disgust sensitivity connecting to conservative voting patterns (Shook et al., Reference Shook, Oosterhoff, Terrizzi, John and Brady2017) and food and health policy attitudes (Clifford & Wendell, Reference Clifford and Wendell2015) and conservatives avoiding disgusting images (Oosterhoff et al., Reference Oosterhoff, Shook and Ford2018). Hunger also alters policy decisions among both citizens (Aaroe & Petersen, Reference Aaroe and Petersen2013) and judges (Danziger et al., Reference Danziger, Levav and Avnaim-Pesso2011).
Biological factors such as our genes, brains, sense of smell, and tastes all interact with our identities, affiliations, and political attitudes. Neural mechanisms in tasks not obviously related to politics have nonetheless differentiated partisans and ideologues. The process of food decisions, rather than the decisions themselves, has been argued to reveal political differences (Sayre, Reference Sayre2011). Thus, we set out to investigate whether the neural mechanisms involved in making decisions about food purchases differed between Democrats and Republicans in two experiments.
Two functional brain imaging experiments of food purchase choices
Participants
One hundred healthy, right-handed, English-speaking, non-vegan, non-lactose intolerant adult participants (ages 18–55; mean = 31 years; 49 females) from the Kansas City metropolitan area underwent fMRI scanning at the Hoglund Brain Imaging Center at the University of Kansas Medical Center on a 3-Tesla Skyra (Siemens, Erlangen, Germany) scanner. The study collected political, demographic, biometric, and psychographic information from all participants. Seven participants dropped out during the fMRI scanning. Seventeen participants stated their political affiliation as non-affiliated, and eleven participants as “other” party. Their data was excluded from the primary analysis. In the end, this study analyzed 65 participants, among which 40 were Democrats and 25 were Republicans.
The differences in political affiliation between participants in our study were not driven by sociodemographic characteristics. We tested the equality of means for the sociodemographic variables within all four groups of participants (i.e., self-reported Democrats, Republicans, Independents, and Others) and concluded that there are no significant differences between the different groups regarding gender, age, education, income, and race. This finding corroborates the conclusions of Mosier & Rimal (Reference Mosier and Rimal2020), who demonstrated that gender, education, and race are consistent explanatory factors of self-reported dietary habits across all political affiliations (i.e., Democrats, Republicans, and Unaffiliated). We know of only one brain imaging study that has examined unaffiliated voters (Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan and Paulus2020) and hope that future studies will also consider comparisons with independent, unaffiliated, or “other” parties.
Two fMRI Experiments
Two separate experiments were performed: a milk-choice experiment and an egg-choice experiment. For the milk experiment, participants underwent fMRI scans and completed 84 non-hypothetical, binary choices between two milk product images labeled with various prices and the production technologies used. Likewise, for the egg experiment, participants underwent fMRI scans and made 84 non-hypothetical, binary choices between two product images of a dozen eggs labeled with prices and production methods. Participants were given $50 and told that they would be given one of the products they chose during the experiment, with the price of the choice deducted from the payment. In both experiments, participants went home with one of their choices (a gallon of milk or one dozen eggs).
We presented participants with choices where the images showed milk or eggs produced in different ways and at different prices. Specifically, the labels on the images differed according to three experimental conditions for the 84 choices: (a) 28 choices were in the “price condition,” in which two products were produced with the same production method, but the prices varied (between $3 and $7 in $0.50 increments in the milk experiment, and between $0.99 and $4.99 in $0.50 increments in the egg experiment); (b) 28 choices were in the “production method condition,” in which one of the milk products was labeled as either “from a cloned cow” or using “artificial growth hormone,” while the comparative milk was labeled as coming from either a “non-cloned cow” or a cow treated with “no added growth hormone.” Likewise, one of the egg products was labeled as coming from hens that were either “caged hens” or “confined hens,” and these products were compared with either “cage-free” or “free-range.” In the “production method condition,” all choices were offered at the same price, and, finally, (c) the remaining 28 choices were in the “combination condition,” in which the product with a higher price in the milk experiment was either “non-cloned” or “no added growth hormone” milk while in the eggs experiment, the higher price went to the eggs from hens that were not confined.
The pricing used in the combination condition was chosen because non-confinement practices would raise prices for eggs, but growth hormone or cloning would lower milk prices. The combination experiment is the method considered to be the most realistic, as shoppers must decide upon competing products based on a combination of changing factors. Each choice pair remained on the visual monitor until the participant decided. Following each choice, participants were presented with a confirmation screen indicating which selection they had made. The time to make a decision varied both across and within participants’ choices. In order to obtain a consistent image, the confirmation screen was presented no less than 0.5 seconds but no more than 3.5 seconds after the participant made a choice. There were two functional runs in which participants made 42 choices (84 total choices). A fixation cross was presented for 3–15 seconds to jitter the inter-trial interval. The optimal timing of trials was estimated using an Analysis of Functional Neuroimage (AFNI) stimulus timing program (make_random_timing.py) to minimize collinearity issues in the fMRI analysis. The order of presentation of choices from the three conditions was randomized in each experiment.
To simulate real shopping behavior, we used images of standard, plastic-gallon jugs for the milk experiment. Milk from cloned cows had been approved by the FDA but was not on the market at the time of data collection. Milk from cows with artificial growth hormone is available but is controversial (Pollack, Reference Pollack2006). In the egg experiment, all the production practices presented to the participants currently exist in the marketplace. We used images of standard one-dozen-sized cartons that differed only in the price or production method label. Figure 2 provides an example of the types of images that participants saw in the two experiments.

Figure 2. Examples of Images from the Milk and the Egg Experiment.
As Glimcher & Rustichini (Reference Glimcher and Rustichini2004) contended in a position paper on the discipline of neuroeconomics: “People are seen as deciding among options on the basis of the relative desirability of each option” and “[d]esirability is computed and is represented in the brain, and we now have the means to test, measure, and represent this activation.” Varying the prices of foods and asking participants to make decisions among foods offered at different price points while their brain activity is measured is now a standard way of realizing the hopes that Glimcher and Rustichini articulated (see e.g. Kislov et al., Reference Kislov, Shestakova, Ushakov, Martinez-Saito, Beliaeva, Savelo and Klucharev2023; Knutson et al., Reference Knutson, Rick, Wimmer, Prelec and Loewenstein2007). Because the use of new food production technologies involves cost, ethical, safety, and certainty tradeoffs, neuroeconomics researchers studying food purchase decisions have also presented consumers with alternative ways their food is produced to see how that changes decision-making (for a review see Lepping et al., Reference Lepping, Papa and Martin2015; Stasi et al., Reference Stasi, Songa, Mauri, Ciceri, Diotallevi, Nardone and Russo2018).
fMRI data acquisition
Functional MRI data were analyzed using the BrainVoyager QX statistical package with random effects (Brain Innovation, Maastricht, Netherlands, 2004) and corrected for multiple comparisons. Following Martin et al. (Reference Martin, Holsen, Chambers, Bruce, Brooks, Zarcone and Savage2010), preprocessing steps included trilinear 3D motion correction, sinc-interpolated slice scan time correction, 3D spatial smoothing with 4-mm Gaussian filter, and high-pass filter temporal smoothing. Functional images were realigned to the anatomical images obtained within each session and standardized using BrainVoyager Talairach transformation, which conforms to the space defined by Talairach & Tournoux’s (Reference Talairach and Tournoux1988) stereotaxic atlas. Functional scans were discarded if participants moved more than 4 mm along any axis (x, y, or z). Two runs were discarded due to excess motion, and three participants were unable to complete the task, leaving a total of 92 runs. As in Moll et al. (Reference Moll, de Oliveira-Souza, Eslinger, Bramati, Mourão-Miranda, Andreiuolo and Pessoa2002) and Martin et al. (Reference Martin, Holsen, Chambers, Bruce, Brooks, Zarcone and Savage2010), activation maps were analyzed using the parametric statistical methods of Friston et al. (Reference Friston, Holmes, Worsley, Poline, Frith and Frackowiak1995) (included in the BrainVoyager QX software). Blood oxygenation level-dependent (BOLD) activations during the choices were conducted using multiple-regression analysis (general linear model). Motion parameters were included as nuisance regressors. For the first-level analysis, regressors representing the decision phase (i.e., stimulus onset time to participant choice with an average duration of 2.7 seconds) for the experimental conditions of interest (e.g., price, production method, and combination) were modeled with a hemodynamic response filter and entered into the multiple-regression analysis using a random-effects model. In addition, the feedback phase (i.e., confirmation of feedback, 0.5 seconds) was included as a regressor of no interest. Regressors were modulated for the decision duration. However, there was no amplitude modulation or orthogonalization. Mean percent signal change values were extracted for each individual for each condition as described below to examine associations between product choices for each experiment.
No studies have yet examined the influence of political preferences on food choices during a neuroimaging experiment. As such, we had no specific a priori regions of interest related to politics during our food choice experiment. We therefore conducted a whole-brain analysis examining contrasts between self-reported Republicans and Democrats in blood oxygenation level-dependent (BOLD) activations from the price choices, production method choices, and combination choices. In this analysis, we subtracted the BOLD activation in the baseline condition averaged across voxels in the cluster of the whole-brain analysis from the choice (price, production method, or combination) condition. This removes the fixation effect so that the remaining BOLD activation would be consistent across participants. We further used a contrast method of two different tasks for extracting the BOLD activation and used Monte Carlo simulation to determine the threshold of 14 voxels (k = 14) at p < 0.05 and alpha of 0.01. To address concerns highlighted by Eklund et al. (Reference Eklund, Nichols and Knutsson2016), we took a number of steps such as using this family-wise error correction to create a more conservative determination of statistical significance. Along with our more conservative measures of the BOLD variables, our project has a relatively large sample for an fMRI study. To check for spurious BOLD extraction, we further test the fitness of our BOLD variables in a logistic regression model of political affiliation.
Results and Discussion
Summary statistics for behavioral choice data
The summary results of the food choices are in Table 1. For the milk and egg combination choices, there is no significant difference between Republicans and Democrats in the average number of choices for the various milk or egg conditions. Thus, food choice itself does not reveal political parties in these experiments. Sayre (Reference Sayre2011) argues that it is not the food choice that reveals political differences but how one makes decisions about food. The finding of significantly different brain activation by political parties during the decision-making process may suggest that the participants are using different underlying thought processes when presented with the choices.
Table 1. Summary Statistics of the Number of Choices Made in the Milk and Egg Combination Experiments

Whole-brain analysis
Table 2 shows the brain regions with associated Brodmann areas where there were significant differences between Republicans and Democrats in each experimental condition (p < 0.05). Three of the areas listed in Table 2 are of less interest for the present work because of the lack of research linking these areas to issues of self-reflection, rationalization, emotion, politics, food choices, or behavioral or economic valuation. These areas are the middle temporal gyrus, the parahippocampus, and the superior temporal lobe. All three of these were active during the milk experiment only. The parahippocampus cortex is known to be associated with memory, especially encoding and retrieval of visual scene stimuli such as landscapes (Aminoff et al., Reference Aminoff, Kveraga and Bar2013). The middle temporal gyrus and the superior temporal lobe are known to be important for the comprehension and recognition of words (Booth et al., Reference Booth, Burman, Meyer, Gitelman, Parrish and Mesulam2002). Harpaz et al. (Reference Harpaz, Levkovitz and Lavidor2009) also suggest that the superior temporal lobe plays a role in processing the subordinate meanings of ambiguous words. In the milk experiment, the labels informed participants of the usage of cloning and hormones, which are arguably more ambiguous than the cage/cage-free type labels in the eggs experiment. Because of the lack of related research linking these regions to areas other than word or image recognition, we are inclined toward skepticism as to their usefulness as general indicators of political preferences. We instead focus on the areas that have been documented in other research studies to be relevant to political preferences, as discussed above: the ventromedial prefrontal cortex, insular cortex, premotor/supplementary motor area, precuneus, and superior frontal gyrus.
Table 2. Results from Whole-brain Analysis: BOLD Responses to Contrasts of Interest (p < 0.05)
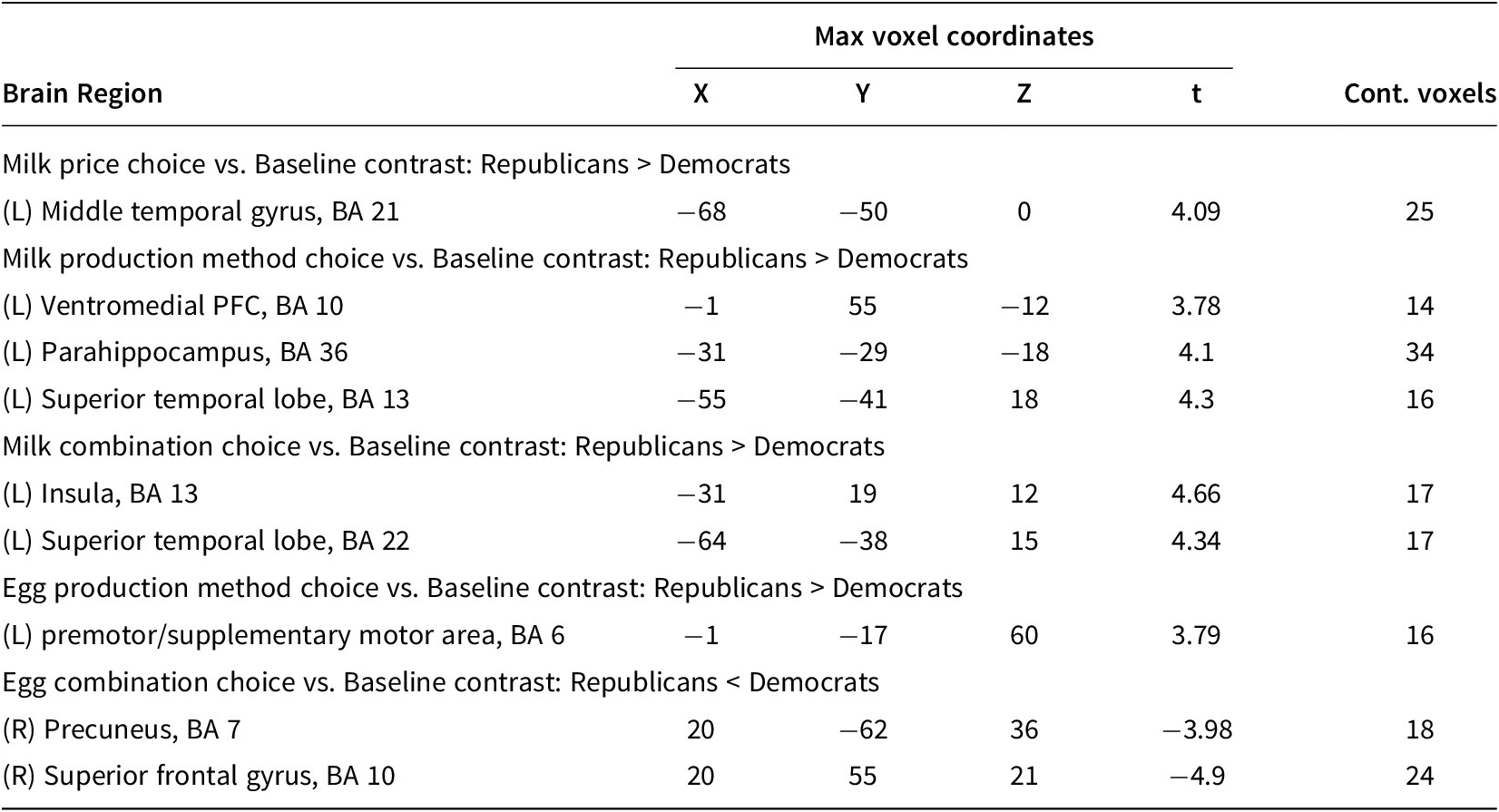
Notes: BA = Brodmann Area.
Figures 3 and 4 illustrate significantly different brain activation by political parties shown in red (greater activation) and blue (less activation). Figure 3a shows the significant activation observed in the left ventromedial prefrontal cortex (vmPFC) for the milk production method condition relative to the baseline condition. The vmPFC is a region involved in processing and evaluation (Ruff & Fehr, Reference Ruff and Fehr2014), associated with self-reflection and self-referential processing (Kelley et al., Reference Kelley, Macrae, Wyland, Caglar, Inati and Heatherton2002; Macrae et al., Reference Macrae, Moran, Heatherton, Banfield and Kelley2004), as well as an area related to the valuation of items, monetary or otherwise (Levy & Glimcher, Reference Levy and Glimcher2012) and has been implicated in previous research on politics (Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006; Mitchell et al., Reference Mitchell, Macrae and Banaji2006; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009).
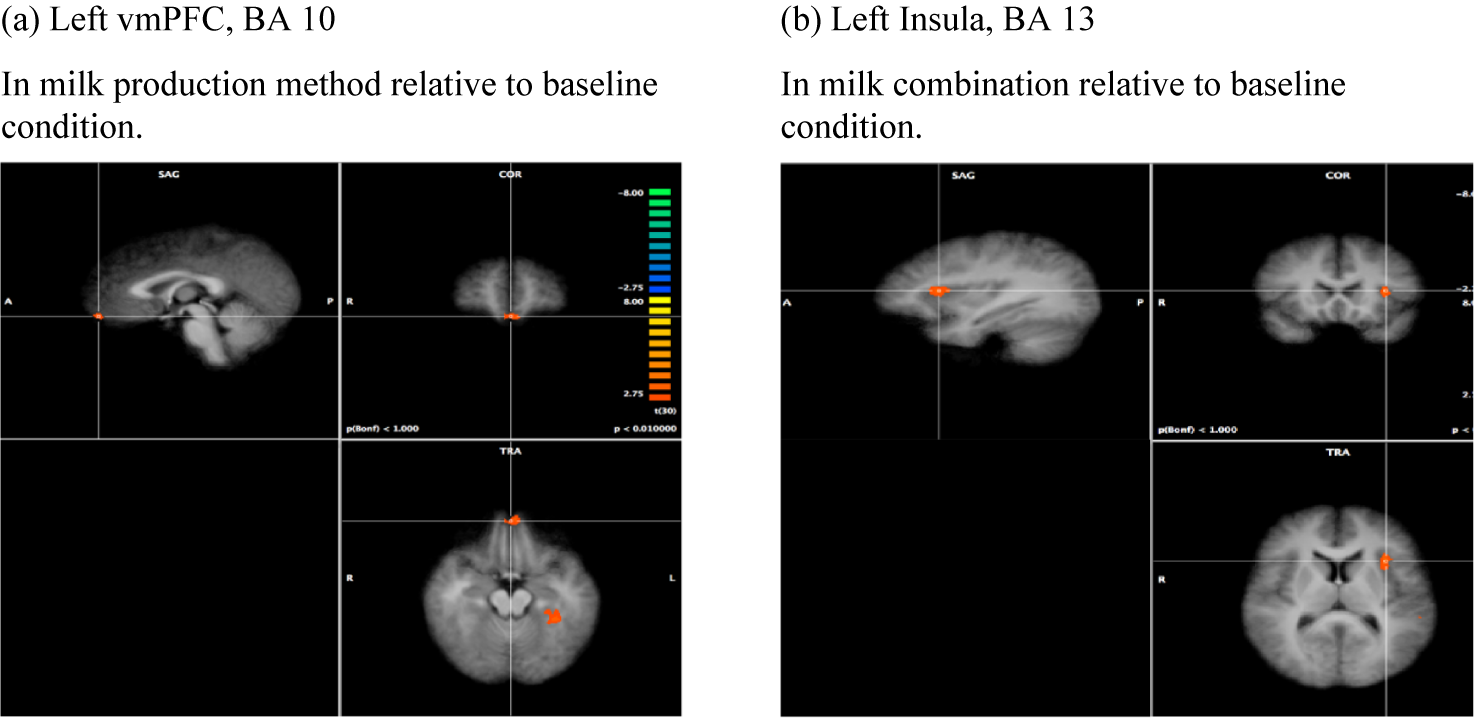
Figure 3. Whole-brain analysis in the milk experiment: Republican-Democrat contrasts.
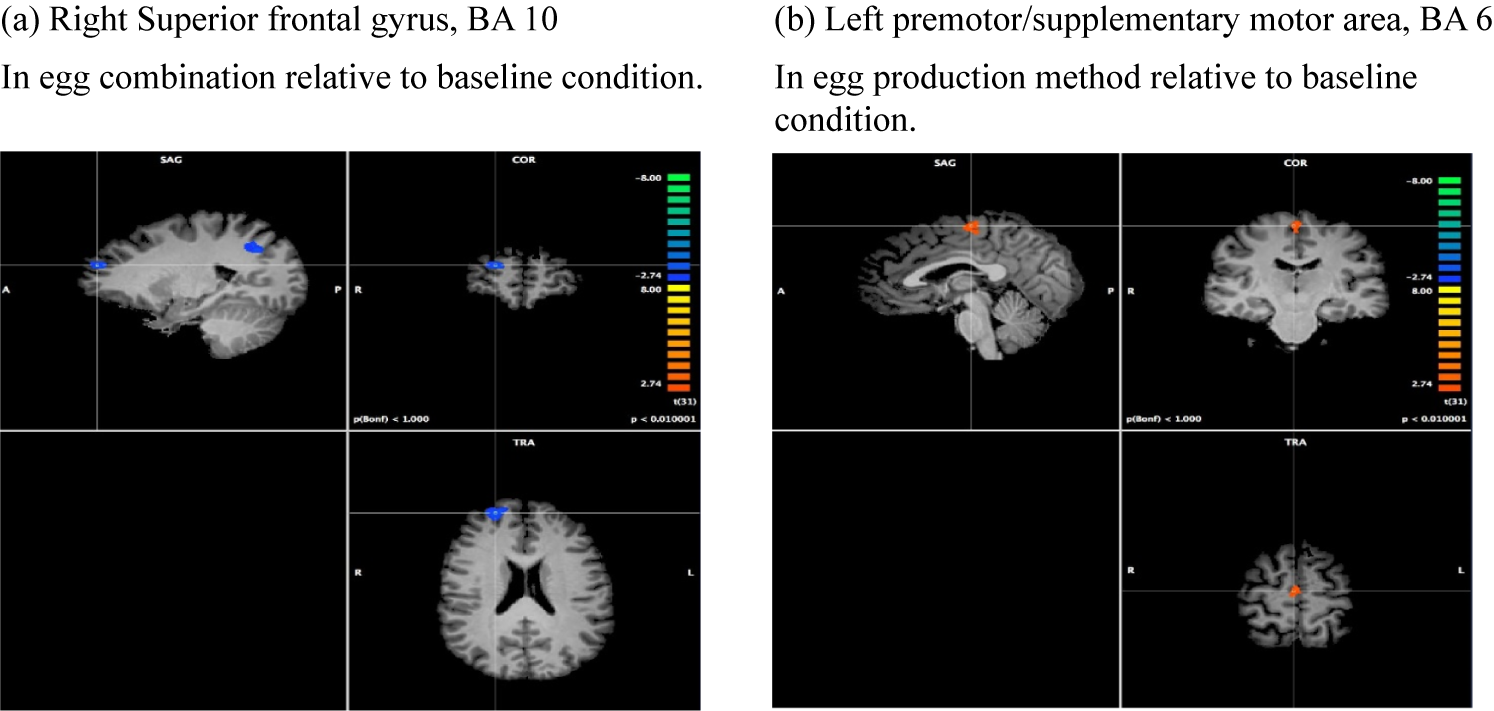
Figure 4. Whole-brain analysis in the egg experiment: Republican-Democrat contrasts.
The combination condition decision-making is the most similar to real-life decisions where attributes like labels and prices vary among food choices. As Table 2 shows the left insula (also in Figure 3b) shows significantly stronger activity in Republicans than Democrats in the milk combination condition relative to the baseline condition. The insula has been frequently implicated in our ability to feel our internal sensations, a phenomenon known as interoception (Haruki & Ogawa, Reference Haruki and Ogawa2021). Bartra et al. (Reference Bartra, McGuire and Kable2013) find that the left insula is associated with a person’s subjective valuation of a good. Insula activity has been found to be an experience-value signal, also associated with pain (Ruff & Fehr, Reference Ruff and Fehr2014) and disgust (Wicker et al., Reference Wicker, Keysers, Plailly, Royet, Gallese and Rizzolatti2003). The neuropolitics literature shows that the insula is implicated in in-group bias (Kaplan et al., Reference Kaplan, Freedman and Iacoboni2007; Westen et al., Reference Westen, Blagov, Harenski, Kilts and Hamann2006) and political ideology (Kanai et al., Reference Kanai, Feilden, Firth and Rees2011; Krosch et al., Reference Krosch, Jost and Van Bavel2021; Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013).
In the egg combination condition, activity in the precuneus and superior frontal gyrus (Figure 4a) is significantly stronger in Democrats than Republicans. The precuneus is involved with episodic memory (Lundstrom et al., Reference Lundstrom, Petersson, Andersson, Johansson, Fransson and Ingvar2003) but also social cognition, including processing stories (Mar, Reference Mar2011). The precuneus is frequently shown to be active while analyzing political information (Fowler & Schreiber, Reference Fowler and Schreiber2008; Gordon et al., Reference Gordon, Quadflieg, Brooks, Ecker and Lewandowsky2019; Kaplan et al., Reference Kaplan, Gimbel and Harris2016; Moore et al., Reference Moore, Hong and Cram2021). The superior frontal gyrus has been posited as a gateway for directing attention and cognitive resources (Burgess et al., Reference Burgess, Dumontheil and Gilbert2007). In the context of politics, it has been found to be active during the processing of political faces and attitudes in a version of the Implicit Association Test (Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006).
Figure 4b illustrates significantly greater activation observed in the left premotor area (PMA)/supplementary motor area (SMA) for Republicans than for Democrats for the egg production method condition relative to the baseline condition. Our findings may complement Amodio et al. (Reference Amodio, Jost, Master and Yee2007) who used a habitual-tendency Go/No-Go task, finding greater liberalism associated with more responsiveness to new, unexpected, conflicting information and stronger anterior cingulate activity.
Finally, neither Republicans nor Democrats have statistically significant differences in amygdala activity in our study, even though previous studies had shown differences between liberals and conservatives in this particular brain area (Ahn et al., Reference Ahn, Kishida, Gu, Lohrenz, Harvey, Alford and Montague2014; Gozzi et al., Reference Gozzi, Zamboni, Krueger and Grafman2010; Kanai et al., Reference Kanai, Feilden, Firth and Rees2011; Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006; Krosch et al., Reference Krosch, Jost and Van Bavel2021; Pedersen et al., Reference Pedersen, Muftuler and Larson2018; Rule et al., Reference Rule, Freeman, Moran, Gabrieli, Adams and Ambady2010; Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013). One reason may be that previous studies used stimuli that provoked stronger emotional reactions, such as images of politicians or threats of loss or pain. Our experiment portrayed food images for which only text labels and prices on the images differed. Food labels and prices may serve as cognitive information signals, especially in the milk experiment (Kolodinsky, Reference Kolodinsky2008). The amygdala is not as involved in the higher-level cognitive functions like conceptual associations (Jost et al., Reference Jost, Nam, Amodio and Van Bavel2014) but is involved with emotional responses and subsequent decisions. The milk and egg choices in our current experiment may not have elicited a very strong emotional response from participants.
How good is the model fitness for political views based on brain activity?
To evaluate model fitness, we followed the examples of Kanai et al. (Reference Kanai, Feilden, Firth and Rees2011), Schreiber et al. (Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013), Ahn et al. (Reference Ahn, Kishida, Gu, Lohrenz, Harvey, Alford and Montague2014), and Yang et al. (Reference Yang, Wilson, Lu and Cranmer2022) and explored how well the activity in the regions we identified could correctly classify a participant as Republican or Democrat. In Table 3, we show four logit regression models that use the results from whole-brain analysis to evaluate the model fitness of the brain activations for the participant’s political view. In general, all four models do better than a random guess (50%) and find that it is harder to correctly classify Republicans than it is to correctly classify Democrats. Our findings suggest that political orientation might be partially rooted in basic neurocognitive mechanisms that occur even when the choices are non-political.
Table 3. Logit Models’ Fitness for Political View (Republican = 1)

Notes: ***, **, and * indicate significance at the 1%. 5% and 10% levels, respectively.
Specifically, Model 1, which uses left insula activity in the milk combination condition relative to the baseline condition, achieves an overall correct classification accuracy of 78%. Compared with Model 1, Model 2 adds the vmPFC activity in the milk production method condition relative to the baseline condition. However, Model 2 does not improve the overall rate of correct classifications compared with Model 1, even though research commonly finds that the vmPFC activity is different in liberals and conservatives (Mitchell et al., Reference Mitchell, Macrae and Banaji2006; Knutson et al., Reference Knutson, Wood, Spampinato and Grafman2006; Zamboni et al., Reference Zamboni, Gozzi, Krueger, Duhamel, Sirigu and Grafman2009). Model 3 includes as classifiers the areas examined in whole-brain analysis in the egg combination condition relative to the baseline condition and identifies 100% of Democrats correctly. Model 4, which uses a single brain activity variable from the egg production experiment, achieves a rate of correct classification of 76% for political affiliation. These results compare favorably with previous neuropolitics studies (Ahn et al., Reference Ahn, Kishida, Gu, Lohrenz, Harvey, Alford and Montague2014; Kanai et al., Reference Kanai, Feilden, Firth and Rees2011; Schreiber et al., Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013; Yang et al., Reference Yang, Wilson, Lu and Cranmer2022).
Conclusion
We found that when making non-hypothetical, economic decisions about food, Republicans show greater neural activity than Democrats in specific regions of the brain, and Democrats have greater neural activity than Republicans in other regions, yet the ultimate food decisions are not significantly different between the two groups. There is no specific conservative, liberal, Republican, or Democrat “grocery shopping” region of the brain, which means political decisions, economic decisions, and day-to-day decisions such as food choices must be made using the available decision “hardware.” In this exploratory study, we expected that there might be differences in the food purchase decisions correlating with partisanship. When we found no differences in the decisions, it was then surprising that a whole-brain analysis revealed that certain regions showed significant differences between Republicans and Democrats when participants made food purchase decisions concerning milk and eggs. Along with using a very conservative extraction for our BOLD variables, we also examined the model fitness of these variables for political affiliation. Not only do our collected BOLD variables correctly classify political affiliation 76–94% of the time and perform better than a random baseline (50%), but they also outperform the baseline expectation from parental conservatism, as reported in Schreiber et al. (Reference Schreiber, Fonzo, Simmons, Dawes, Flagan, Fowler and Paulus2013)(69.5%) and Yang et al. (Reference Yang, Wilson, Lu and Cranmer2022)(71.5%).
In her famous research on crustacean neural systems, Eve Marder (Marder, Reference Marder2011; Marder & Goaillard, Reference Marder and Goaillard2006; Marder & Taylor, Reference Marder and Taylor2011) discovered that a wide range of distinct neural configurations can nonetheless lead to identical behavior. That particular behavior might be evolutionarily adaptive in the specific conditions, but evolution works on variation (Darwin, Reference Darwin1996 [1859]), and having identical neural systems generating the currently advantageous behavior would make a population evolutionarily vulnerable if conditions change. While Marder’s initial research was at the level of basic neuroscience under laboratory conditions, her more recent work has seen the consequences of actual, rather than merely theoretical, changes in environmental conditions (Marder & Rue, Reference Marder and Rue2021; Schapiro & Marder, Reference Schapiro and Marder2024).
The wild-caught crabs brought into her lab now do not appear any different from the previous generations that she had studied in standard laboratory control conditions, but when exposed to temperature extremes in the lab, tremendous differences have recently arisen. Because most neuroscience research focused on carefully inbred model organisms like mice, flies, and worms, the assumption that all individual organisms use equivalent neural systems to generate equivalent behaviors is essentially baked in as a consequence of the experimental designs (Marder & Rue, Reference Marder and Rue2021). Marder’s reliance on wild-caught animals introduced a natural diversity that enabled her to make important insights into variations in neural systems that would not have been easily seen in typical white lab mice. But it has also turned a basic bench neuroscientist into an inadvertent climate researcher.
In the current project, we observed Republicans and Democrats generating indistinguishable food purchase behavior using distinct sets of neural mechanisms. This is much like the identical behavior of crabs that came from distinct neural systems in Marder’s early work (Marder & Goaillard, Reference Marder and Goaillard2006). The field of political science has historically focused on behaviors like voting, protesting, or responding to survey questions, particularly because it was so difficult to measure processes or subjective states (Converse, Reference Converse and Apter1964), but the neural underpinnings of such behaviors have yet to be fully elucidated.
As Marder’s research shows, however, focusing entirely on behaviors constrains our ability to understand the function of the ‘multiple solutions’ (Marder, Reference Marder2011) that might still generate the same outcomes. If, ultimately, we want to move towards explaining differences among partisans rather than merely describing them, it is critical that we investigate the instances where the underlying processes differ, not merely the behaviors. A classic ‘66 Mustang and a modern Tesla may both drive down the same road at the same speed and make the same turn, but looking under the hood reveals important distinctions. The accumulating evidence that neural differences that are strongly correlated to partisanship or political ideology nonetheless generate identical nonpolitical behavior (Schreiber, Reference Schreiber2018) highlights the limits of merely studying behavior and the importance of understanding why these neurological processes correlate with political differences.
Much of early neuroscience and early political science assumed roughly equivalent mechanisms driving equivalent behaviors. From John Locke (Reference Locke1690) on through the twentieth century’s political behavior research, the emphasis was on the environment and experience of writing on similarly situated blank slates (Pinker, Reference Pinker2002). Likewise, in psychology and neuroscience, the emphasis was on the external stimulus generating the response in carefully controlled experiments with roughly identical lab animals (Skinner, Reference Skinner1938). The tools of basic neuroscience are now revealing the diversity of neural systems in both crabs and humans. Both humans and crabs may exhibit fight or flight responses when threatened, but that fact obscures the diverse neural systems generating those similar behaviors.
In a time when affective polarization is raising the political temperature, some previous brain imaging studies demonstrated identical behaviors coming from distinct neural activity corresponding with party and ideology amid emotionally charged nonpolitical tasks (risk, disgust, pain, etc.) Other studies showed brain synchronization regardless of political affiliation when participants watched neutral documentary videos, but a tendency for brains to polarize along party lines when people viewed contentious political content such as campaign ads, speeches, and debates (Katabi et al., Reference Katabi, Simon, Yakim, Ravreby, Ohad and Yeshurun2023; van Baar et al., Reference van Baar, Halpern and FeldmanHall2021). We use the same brain for all of the activities and choices in which we engage.
Like Marder’s early basic neuroscience research under mundane conditions, this paper has shown that mundane decisions like purchasing eggs or milk can lack emotional potency and political content, yield unremarkable behaviors, and nonetheless enable us to correctly classify someone by party affiliation. If the political climate continues to warm, basic research into the neural mechanisms that differ and yet appear to lie dormant in ordinary circumstances may be critical for understanding increasing affective polarization. Future research will need to examine how brains function during daily decision-making if we are to untangle the complexities of political decision-making.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, DS. The data are not publicly available due to legal and ethical restrictions (e.g. their containing information that could compromise the privacy of research participants.)
Financial support
The present study was funded by a grant from the United States Department of Agriculture (Grant No. 2011–67023-30047, http://www.usda.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interest
The authors have no competing interests.
Ethical standard
The present study was approved by the Social Sciences Institutional Review Board of the University of Missouri–Kansas City (UMKC), as well as the Human Subjects Committee of the University of Kansas Medical Center (KUMC). All participants provided their written, informed consent to participate, the procedure for which was also approved by the aforementioned institutions.









