Introduction
Leishmaniasis is a spectrum of diseases estimated to give rise to over 1.5 million new cases each year in 98 countries, with 367 million people at risk. Clinical symptoms range from the disfiguring skin lesions of cutaneous leishmaniasis (CL) to the often fatal visceral leishmaniasis (VL). Approximately 0.4 million VL cases and 0.7–1.2 million CL cases are estimated to occur each year (den Boer et al. Reference den Boer, Argaw, Jannin and Alvar2011; Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). Estimated mortality ranges between 20 and 40,000 leishmaniasis deaths annually (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012) although these numbers are probably an underestimate, given the lack of surveillance systems and frequency of misdiagnosis.
Human leishmaniasis treatment is based on the parenteral administration of highly toxic drugs, including pentavalent antimonials and amphotericin B. Only one drug, miltefosine, is available for oral administration but the selection of resistant parasites is now a concern. The need for the development of new treatment strategies for leishmaniasis is pressing (Rama et al. Reference Rama, Kumar and Balaji2015).
Tamoxifen is a synthetic triphenylethylene in use for the treatment of oestrogen-responsive breast tumours since 1971. It has also been approved for the treatment of metastatic breast tumours and as a chemopreventive drug in groups with high risk for the development of breast cancer (reviewed in Jordan, Reference Jordan2002). Tamoxifen belongs to a drug class known as selective oestrogen receptor modulators (SERMs). SERMs exhibit tissue-specific oestrogen receptor agonist or antagonist activity. Binding of oestrogen to its receptors induces conformational changes that result in the recruitment of transcription factors and triggers the expression of oestrogen-dependent genes. Several oestrogen responsive genes are implicated in tumoral transformation and/or metastasis. Tamoxifen and other SERMs compete for receptor binding and trigger the recruitment of a distinct set of transcription factors or transcriptional co-repressors (reviewed in Jordan, Reference Jordan1984).
Tamoxifen also exhibits several other effects that are not dependent on the interaction with the oestrogen receptor, such as disruption of membrane physiology by drug accumulation in the lipid phase, inhibition of ATP dependent membrane acid transport, interference in ceramide and cholesterol metabolism, interaction with protein kinase C, modulation of calmodulin activity, and apoptosis induction (O'Brian et al. Reference O'Brian, Ioannides, Ward and Liskamp1990; Wiseman et al. Reference Wiseman, Cannon, Arnstein and Halliwell1990; Cabot et al. Reference Cabot, Giuliano, Volner and Han1996; Mandlekar and Kong, Reference Mandlekar and Kong2001). Tamoxifen has also been shown to alter membrane fluidity in various cell types. In erythrocytes of post-menopausal women (Tsuda and Nishio, Reference Tsuda and Nishio2005) and multilamellar liposomes (Severcan et al. Reference Severcan, Kazanci and Zorlu2000) the drug caused an increase in membrane fluidity, while in liposomes (Wiseman et al. Reference Wiseman, Quinn and Halliwell1993) and breast cancer cells (Clarke et al. Reference Clarke, van den Berg and Murphy1990) a decrease in membrane fluidity was described. The study of interactions of tamoxifen with model lipid membranes showed that the drug increases the bilayer thickness and impairs lipid bilayer integrity (Khadka et al. Reference Khadka, Cheng, Ho, Katsaras and Pan2015).
The activity of tamoxifen against different Leishmania species, such as Leishmania (Leishmania) amazonensis (Miguel et al. Reference Miguel, Yokoyama-Yasunaka and Uliana2008), Leishmania (Viannia) braziliensis, Leishmania (Leishmania) infantum chagasi (Miguel et al. Reference Miguel, Zauli-Nascimento, Yokoyama-Yasunaka, Katz, Barbieri and Uliana2009) and Leishmania (Leishmania) major (Eissa et al. Reference Eissa, Amer and El Sawy2011) has been demonstrated both in vitro and in animal models. However, the mechanism responsible for tamoxifen's antileishmanial activity remains unknown. In this work, we investigated the effects of tamoxifen on Leishmania membranes.
The study was performed with promastigotes for several reasons. Amastigotes, which are the life stage found in the mammalian host, are intracellular parasites of macrophages, living inside the parasitophorous vacuoles. Therefore, the analysis of the membranes of amastigotes is complicated by the environment where they exist. When amastigotes are purified from in vitro cultured macrophages, the procedures involved may result in damage to the parasite and more specifically to their plasma membrane. On the other hand, while there are differences in the membrane composition of the two life stages, there are also many similarities (Goad et al. Reference Goad, Holz and Beach1985; Berman et al. Reference Berman, Goad, Beach and Holz1986), suggesting that traits observed in promastigotes are likely to be extensive to amastigotes. Another consideration is that, in the case of tamoxifen, both amastigotes and promastigotes are equally susceptible to the drug, as shown previously (Miguel et al. Reference Miguel, Yokoyama-Yasunaka, Andreoli, Mortara and Uliana2007).
Material and methods
Drugs
Tamoxifen was purchased from Sigma–Aldrich (St. Louis, MO, USA). Stock solutions of tamoxifen were prepared in DMSO for all experiments.
Cell culture
Promastigotes of Leishmania (Leishmania) amazonensis (MHOM/BR/1973/M2269) were grown in M199 medium (Sigma–Aldrich) supplemented with 10% heat-inactivated fetal calf serum (FCS; Invitrogen) and 0.25% hemin and incubated at 25 °C.
Promastigotes’ viability assay
The viability of L. amazonensis stationary-phase promastigotes was evaluated in culture media or in phosphate-buffered saline (PBS) supplemented with 5 mm D-glucose (PBS + g). For quantification of drug activity, parasites were incubated in the presence of increasing concentrations of tamoxifen, assayed at a 2-fold dilution. Cell viability was assessed using the PrestoBlue Cell Viability Reagent (Invitrogen), according to the manufacturer's instructions. Fluorescence was evaluated in a microplate reader (POLARstar Omega, BMG Labtech, Ortenberg, Germany) (λ ex = 544 nm; λ em = 590–10) after additional 2 h incubation with PrestoBlue. The half-maximal inhibitory concentrations (IC50) were determined from a sigmoidal regression of the concentration-response curves using GraphPad Prism 5.0 software. Assays were performed in triplicate and results were expressed as the mean and standard deviation of at least two independent experiments.
Morphological analysis of parasites under drug treatment was performed by taking aliquots of the parasite suspension in culture media and staining with Giemsa. Imaging of promastigote cultures was performed using a Zeiss AXIOVERT 10 microscope. Images were acquired from cells cultivated in 24-well plates and kept at 25 °C. At the times indicated in Supplementary Videos 1 and 2, plates were examined and filmed, under a 40 × objective, using a Canon G10 Wide 52 mm camera.
Transmission electron microscopy
Leishmania amazonensis promastigotes (2 × 107 mL−1) were incubated with 20 µm tamoxifen for 2 or 22 h at 25 °C in M199 medium, supplemented with 10% FCS. Parasites were then centrifuged for 10 min at 230 g. Fixation of the pellet was performed in 2.5% glutaraldehyde: 4% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) followed by post-fixation in 1% osmium tetroxide, dehydration in acetone series and embedding in Epon resin. Ultrathin sections were obtained in a Sorvall Ultramicrotome, stained with uranyl acetate and lead citrate and observed under a JEOL transmission electron microscope operating at 80 kV. Images were recorded with a Gatan 785 ES1000W Erlangshen camera.
Assessment of plasma membrane integrity
Propidium iodide (PI) was used to assess plasma membrane integrity of L. amazonensis promastigotes. Stationary-phase promastigotes (2 × 107 mL−1) were loaded with 10 µ m PI in PBS + g at 25 °C. Parasites were transferred into black polystyrene 96-well microplate and fluorescence was recorded continually (λex = 530–10 nm; λem = 620 nm) in a microplate reader (POLARstar Omega, BMG Labtech) when tamoxifen at different concentrations was added to the wells. Parasites treated with 25 µ m digitonin were used as a positive control. Untreated parasites and parasites incubated with the highest volume of vehicle (DMSO) were used as negative controls. Three independent experiments were performed, each one with triplicate samples.
Determination of plasma membrane electric potential (ΔΨp)
The effect of tamoxifen on promastigotes’ ΔΨp was monitored by using the fluorescent probe bis-(1,3-diethylthiobarbituric acid) trimethine oxonol [DiSBAC2(3)] (Invitrogen) as previously described by Luque-Ortega and Rivas (Reference Luque-Ortega and Rivas2010). Leishmania amazonensis stationary-phase promastigotes (2 × 107 mL−1) were added to black polystyrene 96-well microplates in PBS containing 0.2 µm DiSBAC2(3) in a final volume of 100 µL per well. The plate was incubated at 25 °C and fluorescence was recorded (λex = 544 nm; λem = 584 nm) every 2 min in a microplate reader (POLARstar Omega, BMG Labtech). After signal stabilization, 5 µ m tamoxifen was added. Valinomycin 5 µ m (Sigma–Aldrich) was used as a positive control. Untreated parasites and parasites incubated with the highest volume of diluent (DMSO) were used as negative controls. To test whether tamoxifen or valinomycin interfered in DiSBAC2(3) fluorescence, the drugs were assayed in the absence of cells. Three independent experiments were performed, each one with triplicate samples.
Evaluation of mitochondrial transmembrane electric potential (ΔΨm)
Changes in ΔΨm induced by tamoxifen were registered by flow cytometry, using the fluorescent probe rhodamine 123 (Rh123) (Sigma-Aldrich) as previously described by Luque-Ortega and Rivas (Reference Luque-Ortega and Rivas2010). Leishmania amazonensis stationary-phase promastigotes (2 × 107 mL−1) were resuspended in Hank's balanced salt solution supplemented with 10 mm D-glucose (HBSS + g) (137 mm NaCl, 5.3 mm KCl, 0.4 mm KH2PO4, 4.2 mm NaHCO3, 0.4 mm Na2HPO4, pH 7.2, 10 mm D-glucose) and incubated with tamoxifen (0.3 to 10 µ m) at 25 °C for 20 min. After treatment, parasites were washed twice in HBSS + g, loaded with Rh123 (0.3 µg mL−1, 10 min, 37 °C), washed twice in HBSS + g and analysed by flow cytometry. Fluorescence emission was quantified using the CytoSoft 4.2.1 software as described above. Parasites incubated with 100 µ m carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, Sigma–Aldrich) were used as a positive control. Untreated parasites and parasites incubated with the highest volume of diluent (DMSO) were used as negative controls. Results were obtained from three independent experiments.
Statistical analysis
Data were compared using one-way analysis of variance followed by Tukey's multiple range test and considered statistically significant when P < 0.05. Statistical analyses were performed using GraphPad Prism 5 software.
Results
Evaluation of plasma membrane integrity
The IC50 of tamoxifen for promastigotes of L. amazonensis treated in culture media for 24 h was determined as 13.48 ± 1.22 µ m. The morphological evaluation of promastigotes treated with 20 µ m tamoxifen in culture medium for various periods of time revealed that up to 2 h, most cells remained intact (Fig. 1). With prolonged incubation times, the morphology changed with round cells appearing to be vacuolized (Fig. 1F–H).

Fig. 1. Morphology of tamoxifen-treated promastigotes. Photographs obtained by optical microscopy of Leishmania amazonensis promastigotes (2 × 107 mL−1). Untreated control (A); parasites incubated with 20 µ m TAM for 30 min (B), 1 h (C); 2 h (D), 3 h (E); 4 h (F); 6 h (G) and 8 h (H).
The morphological and motility changes were also observed by imaging cultures under drug treatment: parasites incubated in culture medium and 10 µ m tamoxifen showed reduced motility from 90 min without general morphological disruption. Parasites then started to aggregate forming groups of cells. Until 8 h post-treatment, mobile cells were still observed, in progressively lesser numbers in the midst of the parasite aggregates (Supplementary Video 1). The same characteristics were observed in cultures treated with 20 µ m tamoxifen (Supplementary Video 2). However, in these conditions, changes were faster and loss of mobility was observed in nearly all cells after 8 h of treatment.
Transmission electron microscopy of parasites treated with tamoxifen confirmed that, after 2 h of treatment, the plasma membrane remained intact (Fig. 2B–D). Mitochondrial damage was observed through swelling and loss of the matrix content (Fig. 2D, star). Promastigotes treated with tamoxifen also presented structures reminiscent of autophagic vacuoles (Fig. 2C and D). After 22 h of treatment, the plasma membrane was still observable in cells that have lost most of their internal organelles and content (Fig. 2E and F).

Fig. 2. Ultrastructural analysis of tamoxifen-treated promastigotes. L. amazonensis promastigotes were left untreated (A) or treated with 20 µ m tamoxifen for 2 h (B–D) or 22 h (E and F). Treated parasites presented mitochondrial swelling (stars), the formation of autophagosomes (big arrows) and intense exocytic activity in the flagellar pocket (thin arrow) No disruption of the plasma membrane was observed. N: nucleus; M: mitochondria; K: kinetoplast.
The fluorescent probe PI was used to evaluate the early physiological events related to membrane integrity in promastigotes treated with tamoxifen. Treated and untreated parasites were labelled with PI, which is non-permeant to intact membranes but taken up by cells with damaged membranes. When cells were treated with 5 µ m tamoxifen no PI uptake was observed until 75 min of incubation. A dose-dependent membrane rupture effect was seen when 20 or 40 µ m tamoxifen concentrations were tested with a progressive increase in PI uptake. Drug concentrations of 10 µ m resulted in membrane damage only after periods longer than 30 min (Fig. 3). The detergent digitonin was used as a positive control for cell permeabilization and resulted in an immediate significant increase in PI uptake (Fig. 3). These assays were performed with cells incubated in PBS + g, and in these conditions, tamoxifen displays an IC50 of 11.34 ± 0.90 µ m after 2 h treatments. Therefore, these results indicated that some degree of membrane rupture is likely to take place as the time in the presence of drug and/or concentration increases. However, at low concentrations (5–10 µ m) the plasma membrane remains intact for at least 30–60 min.
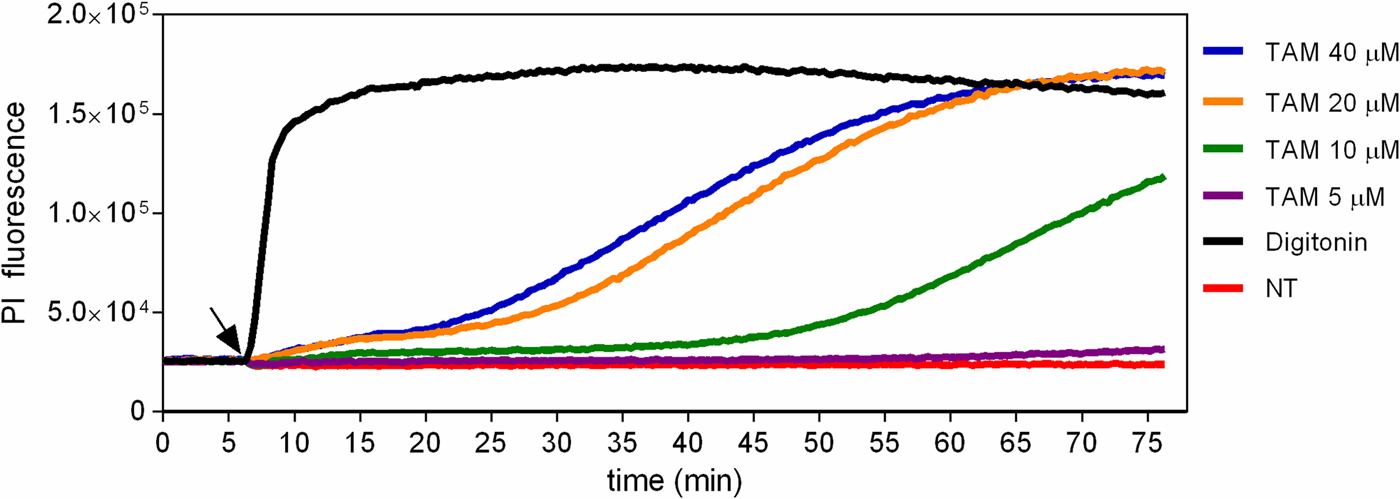
Fig. 3. Evaluation of propidium iodide labelling in tamoxifen-treated parasites. L. amazonensis promastigotes were loaded with 10 µ m PI in PBS + g at 25 °C. Fluorescence was recorded continuously (λ ex = 530–10 nm; λ em = 620 nm) and 5, 10, 20 or 40 µ m tamoxifen (TAM) or 25 µ m digitonin were added to the wells at the time indicated by the arrow. Traces are from one experiment representative of three independent experiments. Untreated parasites (NT) were used as negative control.
Plasma membrane electric potential
To investigate the effect of tamoxifen on ΔΨp, parasites were incubated with DiSBAC2(3) and analysed by fluorimetry. DiSBAC2(3) is a negatively charged dye that moves into depolarized cells (positively charged) and out of hyperpolarized cells (negatively charged) in response to changes in the membrane potential. Once inside living cells the dye binds to lipids and proteins, causing it to fluoresce brightly. Promastigotes treated with 5 µ m of the ionophore valinomycin displayed a sharp increase in fluorescence, indicative of total depolarization (data not shown). We observed that the addition of 5 µ m tamoxifen to cells previously loaded with DiSBAC2(3) induced a rapid change in fluorescence. After 10 min incubation with tamoxifen, fluorescence had increased 2.3-fold over basal levels, indicating membrane depolarization (Fig. 4). Levels of fluorescence attained were indicative of total depolarization, as confirmed by the lack of increase in effect upon further treatment with 5 µ m of the ionophore valinomycin. We also observed that tamoxifen caused DiSBAC2(3) fluorescence to increase even in the absence of cells (Fig. 4). Concentrations of tamoxifen higher than 5 µ m were tested but resulted in greater spurious fluorescence in the presence of DiSBAC2(3) (data not shown).
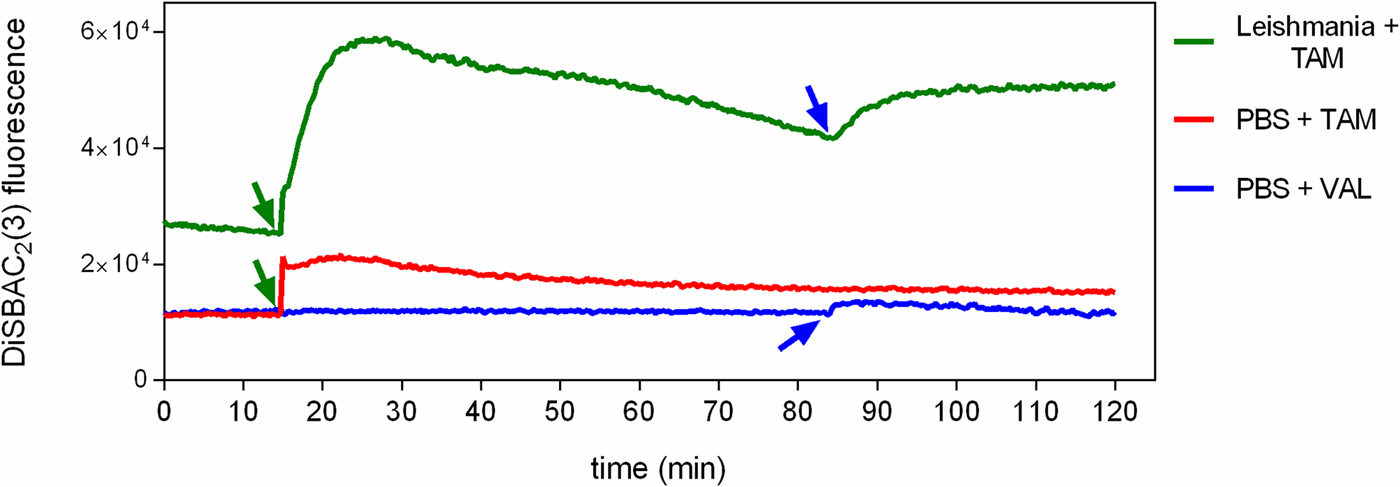
Fig. 4. Effect of tamoxifen on plasma membrane potential. L. amazonensis promastigotes were equilibrated with 0.2 mm DiSBAC2(3) in PBS + g at 25 °C. Fluorescence was recorded continuously (λ ex = 544 nm; λ em = 584 nm) and tamoxifen 5 µ m (TAM, green arrows) or valinomycin 5 µ m (VAL, blue arrows) were added to the wells at the time indicated by the arrows. Traces are from one experiment representative of three independent experiments. The green trace shows the fluorescence in the presence of L. amazonensis promastigotes, while the red and the blue traces show the fluorescence in the absence of parasites.
Mitochondrial membrane electric potential
To verify whether tamoxifen altered the ΔΨm of promastigotes, mitochondrial function was monitored in treated parasites using the fluorescent probe Rh123, a cell-permeant fluorescent dye that is readily sequestered by active mitochondria. Its use relies on the fact that Rh123 accumulates in living cells in a manner that is dependent on mitochondrial membrane polarization. Parasites incubated for 20 min with increasing concentrations of tamoxifen showed a dose-dependent decrease in Rh123 accumulation compared with untreated parasites (Fig. 5). The protonophore FCCP, an uncoupler of oxidative phosphorylation in mitochondria, was used as positive control and caused depolarization of the mitochondrial membrane, with 64 ± 14% reduction in Rh123 accumulation (Fig. 5). The loss of mitochondrial function was accompanied by loss of selective permeability to ions such as calcium both at the plasma as well as internal membranes, as shown by the increased concentration of intracellular calcium in treated parasites (Supplementary Figure 1).

Fig. 5. Tamoxifen interference in Leishmania mitochondrial membrane potential. L. amazonensis promastigotes pre-incubated for 20 min with tamoxifen (TAM) in concentrations ranging from 0.3 to 10 µ m were loaded with 0.3 µg mL−1 Rh123, and the fluorescence level was measured by flow cytometry. Parasites treated with 50 µ m FCCP were used as positive control. Untreated parasites (NT) and parasites incubated with the highest volume of vehicle (DMSO) were used as negative controls. (A) Fluorescence histograms are representative of three independent experiments with untreated parasites (grey filled), 10 µ m tamoxifen (black line), and 50 µ m FCCP (dashed line). (B) Values shown are the mean ± standard deviation of at least three independent experiments. Asterisks indicate significant difference relative to the untreated group (*P < 0.001; **P < 0.0001).
Discussion
Tamoxifen has been suggested as a drug candidate for leishmaniasis treatment based on previously reported in vitro activity against a panel of Leishmania species and in vivo efficacy against infected animals (Miguel et al. Reference Miguel, Yokoyama-Yasunaka and Uliana2008; Reference Miguel, Zauli-Nascimento, Yokoyama-Yasunaka, Katz, Barbieri and Uliana2009; Eissa et al. Reference Eissa, Amer and El Sawy2011). Aiming to explore the mechanism responsible for the antileishmanial activity of tamoxifen, we investigated in the present work its effect on membranes of L. amazonensis promastigotes. Tamoxifen has been shown to be strongly incorporated into biomembranes, to disrupt membrane structure and to induce mitochondrial permeabilization (Custodio et al. Reference Custodio, Moreno and Wallace1998), characteristics that can be correlated with its high hydrophobicity (LogP = 5.93) and are oestrogen receptor-independent.
Membrane integrity can be evaluated through the binding of PI to nucleic acids, while more subtle membrane lesions can be monitored using another fluorescent probe, DiSBAC2(3), which enters depolarized cells, resulting in increased fluorescence. The results obtained indicated that tamoxifen caused a remarkable dissipation of plasmatic membrane potential in the first minutes of incubation. At these early time points and low concentrations, tamoxifen does not disrupt L. amazonensis membrane integrity, as observed microscopically. The observed depolarization appears to be an effect of the collapse of ionic gradients across the membrane. The loss of ΔΨp is due to the passage of ions and small molecules, resulting in equilibration of ions between intracellular and extracellular space. This early effect on parasite's membrane could trigger a series of events that would lead to parasite death. The entry of ions into the cytoplasm driven by electrochemical gradients affects the ΔΨm, which is critical for maintaining the physiological function of the respiratory chain to generate ATP. A significant loss of ΔΨm renders cells depleted of energy with subsequent death. The presented results indicated that tamoxifen causes loss of ΔΨm in a dose-dependent manner and similar results were reported for raloxifene in L. amazonensis promastigotes (Reimao et al. Reference Reimao, Miguel, Taniwaki, Trinconi, Yokoyama-Yasunaka and Uliana2014).
Therefore, we have shown that that tamoxifen induces alterations in plasma membrane potential, with no disruption of its integrity. Tamoxifen also interacts with parasites’ mitochondria, leading to depolarization of its membrane, which results in mitochondrial dysfunction.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/pao.2018.3.
Acknowledgements
We are grateful to Jenicer K. U. Yokoyama-Yasunaka for technical assistance, Dr. Cristiana T. Trinconi for help with experimental procedures, Dr. Antonio Carlos Cassola for helpful discussions, Dr. Noemi Nosomi Taniwaki for assistance with the transmission electron microscopy and Dr. Beatriz S. Stolf for the critical reading of the manuscript.
Financial support
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2011/20484-7 and 2015/09080-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 473343/2012-6). We thank the FAPESP scholarship given to JQR (2011/21970-2). SRBU is the recipient of a senior researcher scholarship from CNPq.







